Patients with spastic paresis often endure prolonged treatment regimens, where each journey is patient-specific and often difficult. Spasticity – defined as increased, involuntary, velocity-dependent muscle tone that causes resistance to movement – is a complex condition that often causes pain, contractures and impairment of basic self-care activities of daily living, such as eating, dressing and attending to personal hygiene. Although the terms ‘spasticity’ and ‘spastic paresis’ are often used interchangeably, the clinical features of spastic paresis are broader, including muscle over-activity, spasticity, dystonia and co-contractions.1,2 We prefer the term ‘spastic paresis’ and have used it throughout this manuscript, but it should be noted that the vast majority of references still use the term ‘spasticity’.
Spastic paresis is associated with damage to the upper motor neurons (UMNs) caused by varying underlying diseases, such as cerebral palsy (CP), multiple sclerosis (MS), stroke, or brain or spinal cord trauma, and is estimated to affect more than 12 million people worldwide.3,4 Despite the substantial impact of spastic paresis on patients’ independence and the burden it places on caregivers, there is a lack of published research on its epidemiology5 and, more importantly, on the patient journey: what does the journey look like, is it clear, and how easily can it be followed in different countries? It is also widely acknowledged that spastic paresis is under-diagnosed.6,7 Its prevalence varies between studies with, as yet, no widely accepted objective measure of spastic paresis. However, it is an accepted principle that if you cannot measure, you cannot manage.
Spastic paresis and the concept of rehabilitation
Making a clinical diagnosis and implementing an optimal clinical management and rehabilitation programme depend on accurately assessing muscle tone, spasticity and functional impairment at multiple time points.8,9 There are several scales commonly used to assess spastic paresis. The Modified Ashworth Scale (MAS) is most often used to assess muscle tone;10 however, it cannot measure either the severity of spasticity or the loss of function that impacts the lives of patients and their families. Therefore, other broader scales or measures that capture changes important to the patients, such as goal setting and attainment, may better reflect an individual’s losses, expectations and achievements from treatment. The Tardieu scale is an example of this, which is more specific for assessing spasticity and which measures the affected limb’s resistance to movement at different speeds using the angle of arrest.11
Each patient has individual needs, and their path through the natural history of disease will vary.12 Typically, patients with spastic paresis expect their treatment to result in a reduction in muscle spasms and a return to normal daily routines.13 Managing adult patients, such as those with poststroke spastic paresis, requires an interdisciplinary approach and timely referral to the appropriate specialists according to the characteristics of the condition.14 The primary parameter of success is the attainment of independence following the re-learning of basic skills such as walking, eating and dressing, and ultimately reintegration into society.15 Therefore, successful rehabilitation depends on a comprehensive but dynamic programme, with appropriate goals being set and assessed at regular time points. This could cover acute inpatient care in a hospital, day-care at a rehabilitation unit of a hospital or outpatient clinic, or rehabilitation in the patient’s home.15
Targeted individual interventions are required; these should be tailored according to whether the spastic paresis is caused by stroke, trauma, MS or CP, and to where the specific impairment is within the body and the central nervous system.8,9,12 Educating physicians and patients regarding appropriate goal setting as well as available therapeutic modalities including pharmacotherapy and non-pharmacotherapy treatment (e.g. physiotherapy or guided self-rehabilitation programmes) is a key requirement to improving patient outcomes.
The patient journey
The primary stakeholder throughout the rehabilitation journey is the patient, followed by his or her caregiver. The specific healthcare professionals (HCP) involved will vary depending on the underlying cause of the UMN lesion and on the patient setting. There are also various types of therapists (physical therapists, occupational therapists, etc.) involved. For example, if stroke is the underlying cause, the specialists involved will usually be neurologists at acute stroke units (within hospitals), and later on, physical medicine and rehabilitation (PM&R) specialists (physiatrists) at inpatient rehabilitation facilities. General practitioners are also likely to be involved, usually later in the patient pathway.
Other individuals involved in the patient rehabilitation journey include those in the financial and administrative sectors, such as insurers, healthcare service decision makers and policy makers. It is essential to consider that different healthcare systems offer different provisions. For example, within the US Medicaid system, although there is dramatic variation between states, there is generally no allowance for adult inpatient rehabilitation. It is important to note that much of the evidence for this paper comes from moderate-to-high income countries; situations may be very different in low-income countries.
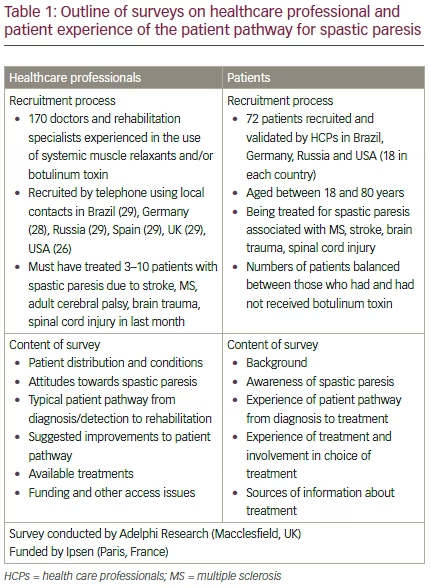
Results of a survey on HCP perceptions of the patient journey and treatment with botulinum toxin type A (BoNT-A) were presented and discussed by the expert group, and subsequently a similar survey was completed by a group of patients with spastic paresis (see Table 1 and the supplementary appendix for more details of the survey).16 The key findings
were that HCPs felt disappointment that spastic paresis was not classed as a separate condition and that there was no clear treatment pathway for patients with this diagnosis. Deficiencies included: referral delays, lack of guidelines, lack of access to BoNT-A treatment, and a high level of bureaucracy. Patients felt that spasticity greatly reduced their quality of life but that all parties lacked clear education and understanding of the treatable symptoms and potential treatment options, leaving patients with significant unmet needs. While patients trusted the key decision makers (usually neurologists) and experienced a smooth treatment process in the acute stages (e.g. stroke), they felt that an earlier awareness of the symptoms that could develop, and all treatment options, would have been welcome to improve the rehabilitation process.
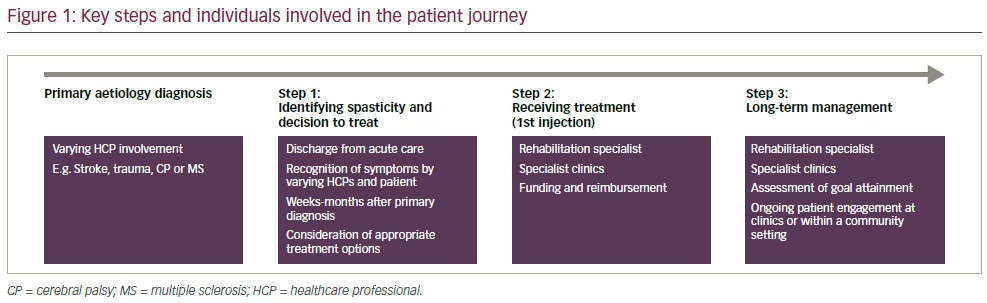
These qualitative surveys and the expert discussion highlighted the need to educate both HCPs and patient groups on the effects of spasticity, the benefits of early spasticity detection, and to raise awareness of all treatment options, including BoNT-A. Here we review the discussions of the experts, together with the results from these surveys, for each stage of the patient journey with regard to the different stakeholders (Figure 1). Although preparation for this manuscript did not include a formal systematic review of the literature, relevant reviews and published studies have been included to inform and support the authors’ opinions on this important subject based on the findings of the surveys.
Step 1 – Diagnosis and decision to treat
The patient
The initiation of treatment in spastic paresis is influenced by several factors related to the underlying cause of the spasticity (Figure 2). Spastic paresis seen in MS is a sign of disease progression, and 84% of patients with MS will experience at least one episode of spasticity.7 Patients with MS often simply accept spastic paresis as part of their condition not realising that it is treatable. By contrast, CP is treated from childhood, and continual assessment is needed, particularly at developmental milestones (e.g. transitioning from adolescence to adulthood), if treatment is stopped, or if follow-up is lacking or inconsistent.
In stroke patients, there is a typical series of adjustment phases, from shock and dissimulation, to adjustment and acceptance.17 Not all patients who have a stroke or traumatic brain/spinal cord injury will develop spastic paresis initially. Therefore, many patients are discharged from acute units with no knowledge that they may develop spastic paresis within 12 months and that it can be treated.18 Furthermore, popular patient information websites, such as www.think-ahead.org.uk, neglect to include any information about the possible development of spastic paresis or potential treatment options. It has been estimated that approximately 20% of patients who experience a stroke develop spasticity after 3 months, and up to 40% develop it after a year.16,19 The predictive factors for developing spastic paresis after stroke include: the presence (and worsening) of paresis in the affected limbs, with more than two joints affected by increased muscle tone; poor motor control on Day 2; MAS ≥2 within a median of 6 weeks after stroke; left-sided weakness; and tobacco use.18,20,21 Community-based HCPs must be watchful for signs and symptoms of spastic paresis so that the patient can be referred for treatment.
In terms of treatment plans, there are a number of options, including combinations of oral and injected medications, use of orthoses, and regular physical therapy. Careful assessment of which symptoms of impaired motor function are limiting the individual patient is essential when selecting the appropriate treatment.22 However, barriers to the patient receiving appropriate treatment include an apparent lack of understanding of the terminology used by HCPs, and patients may also decide against treatment if they are given inadequate information.23 Clearly, educating patients and caregivers is crucial and there needs to be a clear communication pathway.18 Patients also need to be made aware of factors that may aggravate the problem, which include, for example, bad positioning, tight shoes or heavy bed clothes.24
A pertinent example of a patient pathway for referral is the National Institute for Health and Care Excellence (NICE) Stroke Pathway [NICE CG162], involving the patient, caregiver and the multidisciplinary team, which may include neurologists and consultants in PM&R.25 Despite the existence of guidelines such as these, generally, there was a consensus that patients are unable to recognise treatable symptoms or to understand the treatment options available to them. Patient and caregiver education is essential in enabling them to recognise the symptoms of spastic paresis and its comorbidities and precautions, as well as to encourage patients to actively participate in the treatment process (e.g. self-rehabilitation guided by their HCP).24,26
Healthcare professionals
A major challenge for interdisciplinary healthcare teams involved in treating spastic paresis is that the pathway from diagnosis to the decision to treat is highly variable, depending on the underlying condition, and many different professionals will be involved. It is not currently known how many physicians actually adhere to guidelines and treatment algorithms in diagnosing and treating spastic paresis.
To facilitate decision making in terms of predicting the outcome of clinical and administrative choices, the value of simulation modelling has been demonstrated in several studies.12,27,28 However, there remains a lack of highly qualified staff specialising in spastic paresis. Clear communication pathways between HCPs and patients and their caregivers would help to facilitate this.
Patient-centred goals are paramount
Patients should not be seen from just a medical perspective. It is necessary to define why and how a particular patient is to be treated, and to have a patient-centred goal in mind.14,26 Spastic paresis should be treated if it interferes with functioning, positioning, or comfort, and it is advantageous that patients should be treated early unless there is a good argument against it. Ideally, treatment should be initiated before muscle or tendon shortening develops. Patient-centred goal setting is fundamental for successful rehabilitation, for patients’ willingness to continue treatment and to define the best intervention(s) for the patient.29 Without this, patients may be referred for a particular form of rehabilitation therapy out of habit, rather than because there is evidence to demonstrate that it will alleviate symptoms and restore function. Such failure to improve the patient’s wellbeing risks demoralising the patient and is a waste of resources.
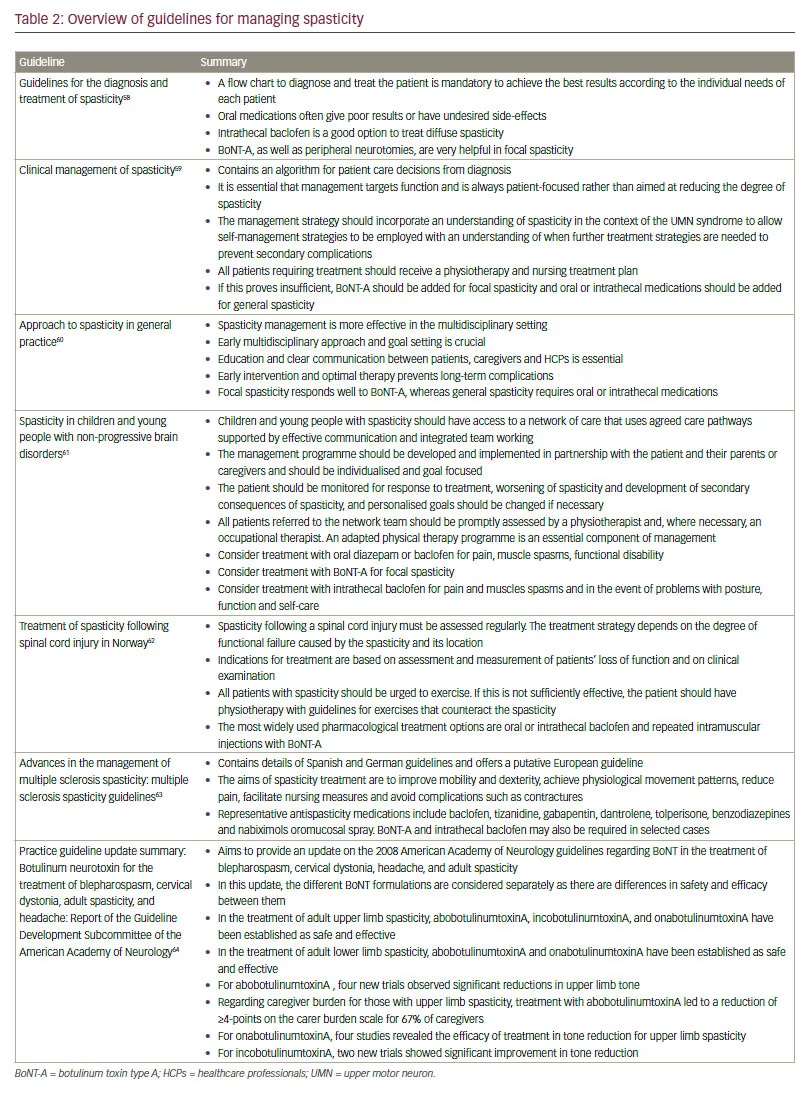
Guidelines for the treatment of spastic paresis emphasise the need for HCPs and patients to collaborate in setting goals for rehabilitation and in defining success criteria for achieving the goals. This includes using language that the patient can understand to describe their symptoms and their treatment.23 Current guidelines for the management of spastic paresis in different aetiologies are summarised in Table 2.
At all stages in the diagnosis and treatment of spastic paresis, the priority for HCPs should be to enable patient collaboration. However, there are challenges involved, and some physiotherapists (and other non-specialist health carers) have reported collaborative goal setting as ‘requiring significant effort, being complex and incredibly difficult’.30 In goal setting, it has been established that at least one standardised outcome measurement should be used.8
Treatment options
In terms of treatment options for spastic paresis, oral medications such as baclofen, tizanidine, dantrolene or benzodiazepines are well established and are the most common treatment of choice, with baclofen being the primary choice (except where stroke is the underlying cause), as they are regarded as easy to use and non-invasive.31,32 There is no evidence, however, that they have any effect on function, and it appears that there may be some instances of oral treatments being used even when they do not constitute the most appropriate therapy.31,33 These drugs may be unsuitable for use in patients with stroke because of the risk of sedation and lowering of the seizure threshold associated with their use.14
The introduction of BoNT-A has had a significant impact on the treatment of focal spasticity in the upper limbs, and it is widely viewed as a welltolerated and efficacious treatment option (when used appropriately, based on the relevant country-specific guidance and applicable summary of product characteristics) in conjunction with physical therapy programmes.25,34–38 Preliminary studies suggest that it is best delivered early, when spastic paresis first sets in, in order to minimise the development of secondary complications, such as contractures.39 It is important to note that patients must also exercise and receive physical and occupational therapy, as long-term disuse can induce muscle atrophy.
Despite evidence of the effectiveness of injectable medications (e.g. BoNT-A, phenol, alcohol)34,35 the authors believe that there exist certain preconceptions and beliefs around their use, which was also reflected in the survey responses. These notions are diverse, and in some cases conflicting. Some survey responders considered them as an option only when oral medications are unsuitable, or when stronger, second-line therapy is needed for more severe cases. Others viewed them as being completely ineffective or offering only very small benefits. Many viewed their role as being limited to patients with focal spasticity, particularly in the case of BoNT-A.37 There is a need, therefore, to review and in some cases challenge these perceptions. Indeed, it was agreed that when patients are not directed to the right specialist to guide their treatment journey, they often remain oblivious of these alternative injectable treatment options and may continue to take inappropriate medications for an extended period. However, there are challenges around addressing these barriers, such as the insufficient numbers of trained professionals in current practice who are capable of administering such injections. Injection techniques also vary between trained practitioners.40 In fact, a recent survey found that nearly half of patients have to wait more than a year before receiving their first BoNT-A injection.13
It has been shown that coaching patients to perform daily selfadministered, rapid alternating stretches can improve spastic paresis by gradually lengthening muscles and reducing their stiffness.41,42 Schemes in which patients and healthcare practitioners jointly set realistic goals for the improvement of symptoms have been shown to be more motivational than standard rehabilitation programmes.42 In addition to oral and injectable medications and guided selfrehabilitation, other treatment options include intrathecal baclofen, physiotherapy, occupational therapy, and surgical treatments (as a final option). These different treatment options are discussed in more detail in the guidelines in Table 2 and in other review papers.32,43–45
Financial and administrative barriers
A coherent and clear set of options for a patient treatment pathway, guided by overarching treatment guidelines, would need to incorporate country-specific financial and administrative aspects to optimise the patient treatment journey. A US study found that constraints on decision making caused by the health insurance model disrupted the process, resulting in delays in the patient’s diagnostic and treatment journey.46 Furthermore, another study, which analysed data from the 2005 Behavioural Risk Factor Surveillance System (BRFSS) survey on stroke survivors in 21 US states, found that only 30.7% received outpatient
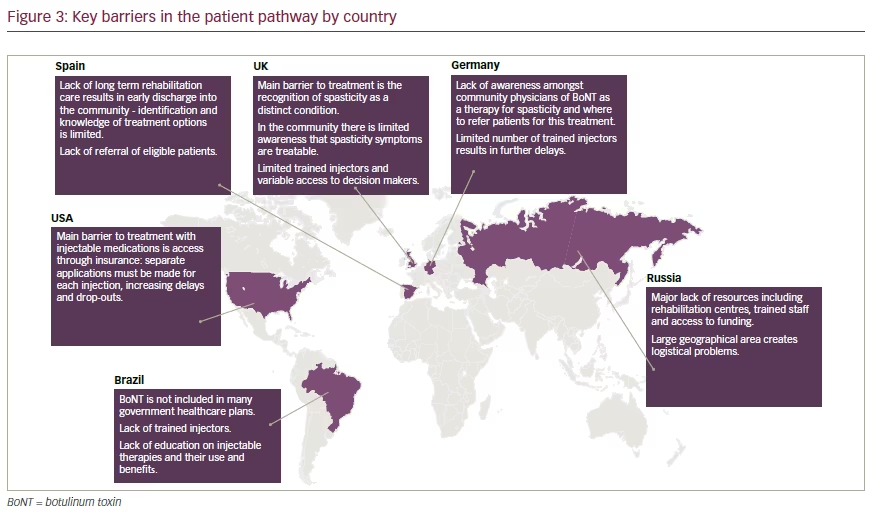
rehabilitation, possibly due to a lack of, or inadequate access to, rehabilitation clinics or centres and resources.47 In Germany and Russia, despite evidence of its efficacy and safety, there are several barriers to the implementation of BoNT-A therapy, including: a lack of awareness of guidelines or agreement among HCPs; the need for country-wide provision of services in every geographical area; the costs associated with BoNT-A injections and rehabilitation overall; and the required staff time both in skill development and patient interactions.48,49
There is a global lack of specialist care and rehabilitation units, and financial constraints persist in terms of insurance and government funding. In some countries, patients who are suitable for treatment are receiving mixed messages as, despite information stating that BoNT-A treatment for spastic paresis is covered by insurance plans, they do not actually receive the treatment because there are not enough specialist centres.8 This may have serious consequences, as it has been found that frustrated patients and caregivers who are not receiving enough information on accessing rehabilitation options seek out, and pay for, inappropriate services.8
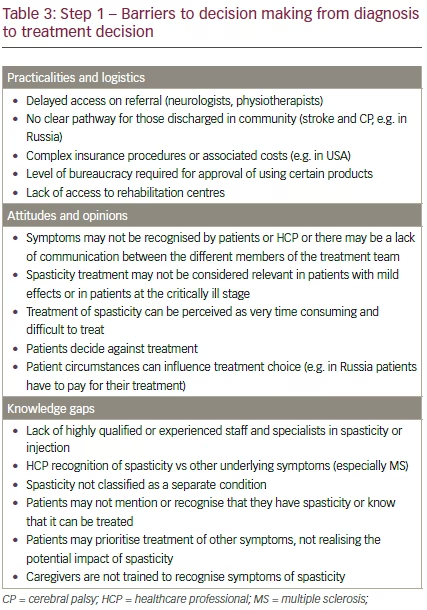
Although reimbursement for BoNT-A is limited in many countries due to perceived cost barriers, it has been shown that it may actually be more cost-effective than oral regimens.26 The lack of information on the global impact of spastic paresis (as measured in terms of morbidity, mortality, quality of life and economic burden) is largely due to a lack of robust data addressing these topics.5 Further research is therefore needed to guide HCPs, patients, and policy makers on the benefits of fully understanding and managing spastic paresis.5 In addition, BoNT-A may simply not be available in the patient’s country or locality. A summary of the barriers identified in the discussions to the first stage of the patient journey are shown in Table 3.
Step 2 – Treatment
The patient
Treatments for spastic paresis include exercise and stretching, which may be used in combination with medication. ‘Contracts’ between therapists (who pledge to deliver an effective rehabilitation programme) and patients (who commit to following the programme) are motivational and effective.42 The European Stroke Organization and The Stroke Foundation of Australia recommend that BoNT-A should be considered alongside rehabilitation therapy for post-stroke spasticity. The Australian guidelines note that intrathecal baclofen is effective but rarely used.50,51
Once the decision to proceed with BoNT-A treatment has been made, patients themselves may be hesitant in agreeing to start or continue with treatment. This may be for a variety of reasons, including innate aversion to injections, fear of treatment with a ‘neurotoxin’, or because they perceive it as a cosmetic procedure.
Healthcare professionals
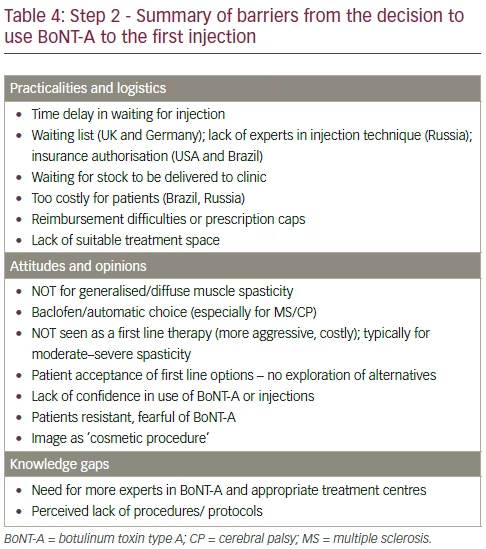
From a HCP perspective, barriers to optimal BoNT-A treatment may include the influence of institutional or personal bias, a lack of knowledge or skill, or a failure to use best practice procedures (Table 4).52 As discussed previously, patient-centred goal setting should form a central part of BoNT-A treatment combined with other physical therapies. These could be augmented in several ways: developing individual best practice strategies for different goal categories (such as pain or function);28 broadly changing the attitude among HCPs towards neurotoxin therapy; and developing more comprehensive education programmes to train future injectors.9
The decision as to when patients should actually start treatment depends largely upon the underlying cause. For patients with brain and spinal cord injuries, or who have experienced a stroke, the initial focus of the interdisciplinary team must be to save the patient’s life. Once the patient’s condition has been stabilised, the team must plan for the patient’s rehabilitation. In the UK and USA, some facilities discharge patients from acute care with ‘rehabilitation prescriptions’ that enable them to rapidly access the most appropriate rehabilitation therapy without having to organise this for themselves.53 This is, by no means, a universal practice in the countries surveyed in the workshops – many patients have long waiting times for treatment, receive inappropriate treatment or end up with no treatment at all because it is difficult to access, is in short supply, or because it is not reimbursed.
Financial and administrative considerations
In this step, financial and administrative considerations are introduced along with those from patients and HCPs. Financial and administrative barriers to the initiation of BoNT-A treatment in practice is different from the barriers associated with other treatments (e.g. oral medications) as BoNT-A is perceived as a more expensive treatment option, including the time delay in waiting for the injection (which may be due to waiting lists, limited numbers of trained injectors, or delayed approval of treatment), as well as cost issues similar to those seen in step 1 (Table 4),8 BoNT-A has, however, been shown to be a cost-effective treatment option.54
A summary of the key discussions regarding step 2 of the patient journey (the initiation of treatment) are shown in Table 4.
Step 3 – Long-term management
Patient factors
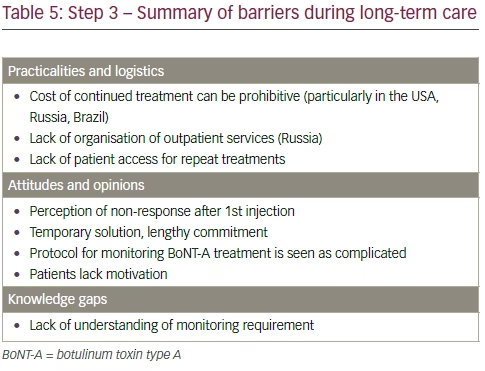
Rehabilitation after an UMN lesion is a lifelong process. However, many patients contend with insufficient outpatient rehabilitation and monitoring in the long term (Table 5).8 Although physical therapy (stretching, positioning and splints) remains the mainstay of the management of spastic paresis,36 the goals of physical therapy are often not well defined, leading to patients feeling unmotivated and disheartened, which may mean they decide to discontinue their rehabilitation. Variations in treatment frequency or reliance on multiple therapists can also affect the patient’s confidence in the therapy and their perception of its usefulness. Without adopting guidelines to establish the expected level of input from the patient and the anticipated outcome, it is difficult to define or recognise a successful treatment programme.
Healthcare professionals
The treatment process for injectable medications is often seen as overly complex. Ideally, HCPs should conduct frequent patient-driven assessments with the appropriate therapists in order to assess the effect of BoNT-A and establish a patient-driven self-management pathway with commitment from all parties.8 The usefulness of registries, such as the The Paul Coverdell National Acute Stroke Registry (PCNASR)55 and the North American Research Committee on Multiple Sclerosis (http://www.narcoms.org/) should also be mentioned for their value as tools to collect data, and to monitor and track long-term care, with the aim of improving patient care.
Financial and administrative considerations Financial and administrative considerations become even more important in the long term management of the patient’s disease. It is a truism that the chances of successful rehabilitation in the long term are largely governed by the availability of resources.54 However, therapy can become intermittent or discontinued as a result of factors such as lack of insurance or reimbursement, as discussed in the previous sections.
In terms of the financial and administrative aspects of long-term management, the cost of continued treatment can be seen as prohibitive, and there can also be constraints in terms of staff time to monitor and
assess patients on an ongoing basis.54 If patients and HCPs are not able to demonstrate continued improvement on medical records with BoNT-A treatment insurance companies may stop paying for the treatment. Alternatively, if there is a change of circumstances at home, patients may have trouble getting to the clinic for their therapy.
These expert discussions, as well as the HCP and patient surveys, highlighted the great variation in the patient journey and challenges faced in different countries. However, they also demonstrated that the barriers are usually the same, just of different magnitudes and with different impacts on the patient pathway (Figure 3). This suggests that global recommendations on how to improve the patient journey for individuals with spastic paresis are needed.
Discussion and conclusion
Spastic paresis occurs as a result of UMN damage, which may be due to stroke, brain trauma, spinal cord injury or CP, as well as underlying conditions such as MS. It should be treated as early as possible in order to prevent muscle or tendon shortening and to thereby improve eventual limb function or position. However, many patients are not treated appropriately.56 The majority of patients are lost to follow-up or their journey is unclear. This is partly because spastic paresis remains underdiagnosed and its burden on patients under-recognised by clinicians. Therefore, education is key to addressing this issue. Currently, there is also no clear treatment pathway for many patients with spastic paresis. There is, therefore, a need for universal guidelines on the optimum treatment pathway to include all treatment modalities, including BoNT-A, which is recommended by several guidelines, but not all.
As rehabilitation is a life-long process, patients have to be trained and guided in order to continue to perform rehabilitation on their own (self-rehabilitation). During treatment there is a need to tailor treatment to the individual patient, as UMN syndrome presents in a very heterogeneous manner. Objective assessments with validated functional outcome tools are essential to measure treatment success and to motivate patients to continue. Patient-centred SMART goal setting (specific, measurable, achievable, realistic and timed), is also important in terms of treatment programmes in order to establish realistic treatment expectations, minimise dropouts, and allow therapy to be tailored to patients’ needs.57
In addition to patient education, there is an overall need for education at all levels across the interdisciplinary team, from clinicians to physical therapists. Trained BoNT-A experts are also needed, following training
in the proper targeting techniques for injection; the consensus at the meeting was that stimulation-controlled or ultrasound-guided injections should always be used to improve the accuracy of injections, improve functional outcomes, reduce side-effects, minimise wastage of neurotoxin, but most of all, to avoid causing patient harm.
Although the challenges to implementing optimal treatment programmes vary by country, patients with spastic paresis face devastating consequences or additional limitations if they do not receive timely and appropriate treatment for their condition. Patients may rely on their caregiver and/or HCP to determine their best treatment options, and to navigate the variable provider and funding hurdles to obtain the best treatment and follow-up.
In order to provide optimal care for patients with spastic paresis, a united and coordinated effort among all parties involved should be in place, including patients, caregivers, medical multidisciplinary team, national legislators, as well as healthcare payors and insurers. There is no mechanism currently in place in any of the countries represented by the authors that incorporates all of these critical entities.
Financing this comprehensive and wide-reaching effort over a prolonged period continues to be a major barrier to achieving optimum treatment for patients with spastic paresis; it has been clearly demonstrated that these patients are under-treated. Although there is a need to ensure that the additional use of injectable medications is clinically indicated, collaboration with industry in a coordinated effort may make it possible to achieve a significant difference for patients who are not currently receiving appropriate treatment. In order to achieve the optimal results for this particularly vulnerable patient population, new and creative, yet controlled, approaches will need to be pursued.
Recommendations
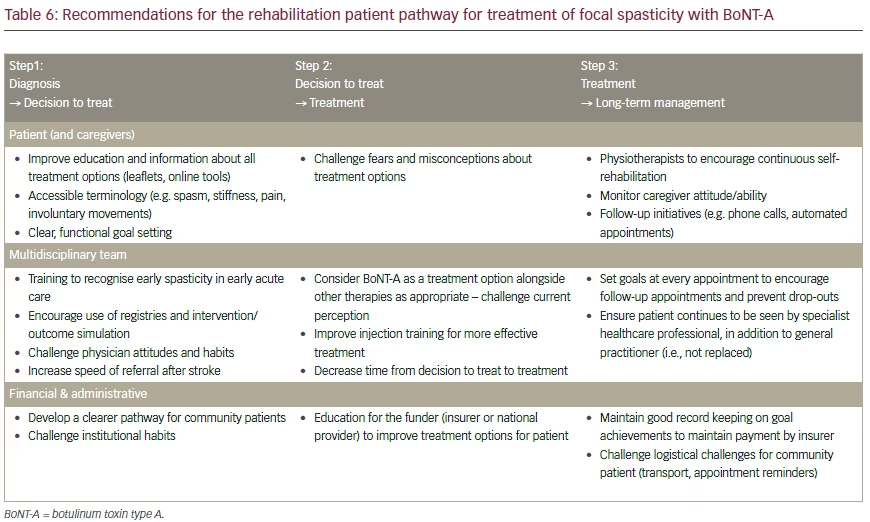
Expert recommendations to address the perceived barriers to the ideal patient journey are summarised in Table 6. It is hoped that by means of further expert panel meetings, and via reports of key conclusions, constructive changes can be expected in the field of spastic paresis management and treatment supported by clearer guidelines.