A tremendous amount of research effort has been put into the understanding and treatment of delayed cerebral vasospasm and its sequelae, namely the development of delayed ischaemic neurological deficits (DIND), since it contributes significantly to overall morbidity and mortality after subarachnoid haemorrhage (SAH), with death continuously occurring in excess of 25 % of patients.1
A tremendous amount of research effort has been put into the understanding and treatment of delayed cerebral vasospasm and its sequelae, namely the development of delayed ischaemic neurological deficits (DIND), since it contributes significantly to overall morbidity and mortality after subarachnoid haemorrhage (SAH), with death continuously occurring in excess of 25 % of patients.1
Paralleled by a growing appreciation for the complexity of the pathophysiology involved, the main focus – after treating symptomatic vasoconstriction with haemodilution and hypertension or local mechanic dilatation2 – has been to introduce newer vasoactive drugs such as endothelin-A antagonists intravenously.3 Though showing a significant reduction in the occurrence of angiographic vasospasm, no change in clinical outcome was observed, and the systemic application of vasoactive drugs was associated with significant side effects. Super-selective intra-arterial application effectively increases cerebral blood flow, but its effect is thought to be transitory, requiring continuous or repetitive infusions.4 Knowing that calcium (Ca) has been identified as a major contributor to post-SAH vasoconstriction, calcium antagonists in particular were under close scrutiny and, consequently, a slow-release implant was developed to be positioned in close proximity to the proximal cerebral vasculature, facilitating an appropriate and effective concentration where it is most needed and thereby combining the beneficial vasodilatory effects while simultaneously avoiding or minimising systemic side effects. Nicardipine is a dihydropyridine-type Ca2+ channel blocker, and nicardipine prolonged-release implants (NPRI) in particular have been investigated in several clinical studies and will be the focus of this review.
Summary of Previous Investigations
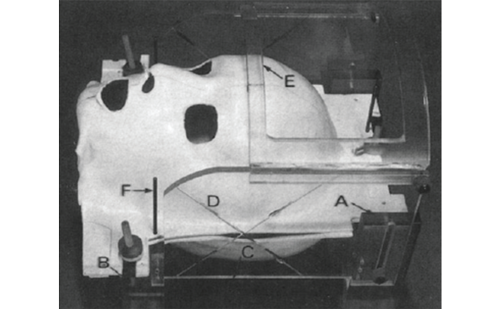
NPRIs were first investigated in a dog model, were pellet application adjacent to the basal vasculature was found to ameliorate vasospasm while maintaining an appropriate concentration of nicardipine via steady release intrathecally over several days.5 Briefly, NPRIs are rod-shaped pellets, measuring 2 mm in diameter and 10 mm in length, each containing 4 mg of nicardipine. Copoly(lactic/glycolic acid) and nicardipine free base are mixed, and the solvent (dicholormethane) is evaporated thereafter. Pellets are then shaped by heat compression.
After extensive investigation using his dog model, Dr Kasuya, the pioneer of intrathecal application of calcium antagonists, successfully translated the setup ‘bench to bedside’ into a clinical context in the autumn of 1999. His first pilot study included 20 consecutive patients after SAH, where computed tomography (CT) scanning showed a Fisher grade III bleed, which generally is thought to be associated with a risk of angiographic vasospasm of 70 % and of DIND of 30 %. NPRIs were positioned in the cisterns surrounding the internal carotid artery, the middle cerebral artery and/or anterior cerebral artery where a thick blood clot suggested the highest likelihood of developing vasospasm.6 Figure 1 shows the pellet placement schematically before an aneurysm is clipped. No complications or neuronal toxicity secondary to the pellet or its components were observed, and chemical meningitis – estimated to be a potential complication in the beginning – was also absent.
Angiography performed on days seven and 12 did not show any vasospasm within those vessels treated with nicardipine, and only one patient developed DIND (5 %). These encouraging observations were followed by a larger, prospective series of patients (n=97), including both low- and high-risk patients as estimated by clinical grade and the amount of subarachnoid blood, treated with or without NPRI in a non-randomised fashion.7 Again, treatment with nicardipine locally led to a decrease in the occurrence of DINDs (6 % versus 11 %), and the proximity of the pellets seemed to be associated with its efficacy in angiographic vasodilation. In this particular study, outcome did not differ between treatment groups, though this is likely attributable to the difference in patient selection, the NPRI group consisting mostly of patients at significantly higher risk of vasospasm (higher Hunt & Hess and Fisher grade on admission). However, the investigators yet again were able to prove the hypothesis of voltage-gate calcium channel blockers effectively forestalling vasospasm if administered in close proximity to vascular smooth muscle for an adequate amount of time.
Although this was neither a controlled, nor a blinded, investigation its findings were complemented by a summary of the first 100 patients treated with NPRIs,8 where NPRI placement completely prevented vasospasm in vessels adjacent to the pellet, but not in more remote regions. Findings of a multicentre cooperative study on NPRIs in Tokyo was then able to confirm these findings yet again in 136 patients, where the rates of angiographic vasospasm (24.6 %), cerebral infarction (12.4 %) and DIND (8.2 %) were found to be decreased significantly.9
Given all of the above-mentioned promising results, our group initiated a single-centre prospective randomised Phase IIa study (NPRI versus no NPRI) in 32 patients.10 All patients were considered high risk (Hunt & Hess grade 3–4, Fisher grade 3) and, when randomised into the treatment arm, received a fixed number of 10 pellets. Digital subtraction angiography (DSA) was analysed in a blinded fashion by an outside institution, and a diameter increase of 115 % was observed in those vessels immediately adjacent to the pellets. Angiographic vasospasm was dramatically reduced in the treatment arm (from 73 % to 7 %, p<0.001), as was the cerebral infarction rate (from 47 % to 14 %, p=0.05) with significant improvement in clinical outcome. Analysing the results of this study in more detail one year after the initial bleed, we found a distinct superiority within the nicardipine group regarding functional outcome as measured by the National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale and Karnofsky Performance Scale, whereas quality of life did not differ significantly between groups.11
A relevant, practical limitation of the above-mentioned studies is the fact that all patients were treated surgically. Given the growing evidence for endovascular approaches as an effective and safe treatment alternative, the feasibility and efficacy of pellet application in those circumstances had to be investigated. As the majority of patients will require either an intracranial pressure monitor and/or an external ventricular drain, an application of nicardipine pellets into the ventricle was developed to address all patients. Previous investigations at another institution have already suggested a beneficial effect of direct application of nicardipine solution into the ventricle;12 however, continuous application was necessary. We initiated a follow-up study, comprising 31 patients after surgical clipping or coiling, where six or 10 NPRIs were applied directly into the ventricle, immediately before ventricular drain insertion.13
Though angiographic vasospasm was again reduced and better outcome with less infarction was observed, the changes were less pronounced than in those patients where pellets could be applied in close proximity to the vessels. Interestingly, efficacy was superior in patients who underwent clipping prior to pellet application, possibly relating to the option of opening of the lamina terminalis and thereby facilitating a more widespread dissemination of the effective agent throughout the cerebrospinal fluid (CSF) spaces.
Discussion
NPRIs are effective in reducing both the incidence and the severity of angiographic vasospasm within the proximal vasculature. This development is paralleled by a reduction in cerebral infarction and DIND. We observe a dose-dependent efficacy, with nicardipine working most effectively when positioned within the basal cisterns.
Efficacy then appears to extend to the contralateral side and even within more peripheral vasculature.10 In high-risk patients, the incidence of angiographic vasospasm can be reduced dramatically, from 70 % to less than 10 %. Overall morbidity and mortality appear to be influenced beneficially, since the infarction rate also decreases substantially. As opposed to previous studies looking at systemic vasodilatory treatment, with NPRI application no significant side effects have been observed so far. Though intraventricular pellet application is feasible and safe, which is of particular interest in patients not undergoing open surgical treatment, its application seems to be less effective,13 as concentration within the area of interest (namely the large, proximal vasculature) may not reach a sufficient level. At this time, open surgical treatment followed by immediate and adjacent placement of calcium-antagonist-containing pellets seems to be most effective in preventing complications from cerebral vasospasm. However, further studies including larger patient groups and investigating different loading doses with NPRIs are required to fully assess the treatment potential, particularly in patients not treated with clipping.
Conclusion
NPRIs effectively forestall the development of cerebral vasospasm and consecutive infarction. Given the improvement in functional outcome, NPRIs represent a most promising option in patients at high risk of vasospasm after SAH and larger, multicentre trials will be needed to further supplement these initial observations.