Cervical dystonia (CD) is a focal dystonia of neck and shoulder muscles that causes neck and shoulder pain, limitation of neck movements, and, sometimes, involuntary head and neck movements. Primary CD is the most common form of adult-onset focal dystonia, with a prevalence of six to nine per 100,000 population.1,2 The peak age at onset is between 40 and 45 years, with females more commonly affected than males (male-to-female ratio 1.4:2.2).3,4 The onset of CD is often insidious, with neck stiffness, limitation of neck movements, abnormal neck posture, and neck pain. Misdiagnosis and delayed diagnosis are not uncommon.
Primary CD has two main clinical forms. The first is characterized by abnormal posture of the head and neck, limitation of head movements, and hypertrophy of overactive muscles, but no involuntary head movements. The abnormal posture can be purely rotational (torticollis), head tilt (laterocollis), head retroflexion (retrocollis), or anteflexion (antecollis), or a combination. Torticollis is the most common among single deviations, while torticollis plus laterocollis is the most common of the combined forms. The second form is characterized by the features of the first type plus involuntary head jerks (myoclonic dystonia). The head jerks are often exaggerated when the patient attempts to move the head toward the direction of movement limitation, and are sometimes seen exclusively at the end of the attempted rotation. In some patients, head movements are oscillatory (dystonic tremor) and difficult to differentiate from the cervical form of essential tremor. Remission occurs in 10–20% of patients, but is generally transient and usually does not last beyond one year.5,6 Approximately 10% of patients disclose a history of CD in first-degree relatives, and 26–52% describe tremor or dystonia in family members.6 CD is distinct from other forms of late-onset dystonia because of pain, which is often described as ‘aching’ or ‘pulling’ and occurs in 70–75% of patients.7,8 In one study the following distribution was reported: neck, 100%; shoulder, 73%; back, 46%; and arm, 15%. The maximum pain is usually felt in the muscles ipsilateral to the side of the chin deviation. Patients learn to perform sensory tricks (geste antagoniste) to improve head posture and decrease neck pain. The most common maneuver is touching the chin on the side of ipsilateral head rotation or tilt. However, pressing the head back against a high chair and holding the occipital region between interlocked fingers may also work. Sensory tricks usually lose some of their effectiveness with the passage of time.
Differential diagnosis of late-onset CD includes a number of secondary causes. Dystonic posture of the neck may arise from cervical spine disease or disorders of the cervico–cranial junction. CD can occur after head or neck injury, and dystonic posture of the neck may be seen during the course of a number of neurodegenerative disorders such as Parkinson’s disease, Parkinson plus syndromes, and Wilson’s disease. In the DYT7 form of genetic dystonia the pattern of dystonia is focal and often affects the cervical region. In some patients with DYT1 dystonia, CD may be the most prominent feature. In younger patients, the work-up should include brain magnetic resonance imaging (MRI), metabolic screening, and—in some patients—genetic testing. In older patients, when there is a suspicion of cervical spine disease one should obtain MRI of the cervical spine and the cervico–cranial junction.
Koukouni et al.10 reported clinical and genetic characteristics of 76 patients with CD with age at onset below 28 years (mean 21 years). The male-to-female ratio was 1.24:1, and there was a family history of dystonia or tremor in 26%. Neck trauma or surgery was present in 17% of the patients. One-third developed dystonia in the contiguous parts of the body. Another one-third experienced partial remission, but dystonia relapsed within five years. The DYT1 gene mutation was negative in all 15 patients who were tested for it. This young-onset CD seems to be distinct from the more common late-onset form of CD in terms of male predominance, higher incidence of family involvement, and more transient remissions.
Primary CD is probably a genetic disorder, but the precise genetic alteration needs to be elucidated. In one German kindred with DYT7 mutation, CD was the prominent dystonic feature.11 In a recent study, the investigators showed that the presence of the D216H variant of the DYT1 gene in the absence of the DYT1 mutation increases the risk for developing CD even in patients without a family history of CD.12
Medical Treatment
The most commonly used pharmacological agents for the treatment of CD are anticholinergic drugs (in particular trihexyphenydil), baclofen, and clonazapam. Greene et al.13 reported the following order of effectiveness for these agents in CD: trihexyphenydil 50%, baclofen 21%, and clonazepam 11%. Unfortunately, high doses of anticholinergics (20–80mg/day for trihexyphenidyl) may be needed to improve symptoms of CD, and such doses often produce undesirable side effects (confusion and blurred vision).14 Clonazepam alone or in combination with botulinum toxins (BoNTs) can be helpful by reducing the sharpness of the head jerks in the myoclonic dystonia variant of CD.
BoNT therapy has changed the life of patients with CD over the past 15 years and remains the most effective mode of treatment. BoNTs comprise seven subtypes, of which only types A and B are in clinical use (type F is effective but has a short duration of action). The antidystonic effect of BoNTs is exerted mostly through inhibition of acetylcholine release from pre-synaptic vesicles via deactivation of SNARE proteins. For type A, this protein is SNAP 25; for type B it is vesicle-associated membrane protein (VAMP, or synaptobrevin). The analgesic effect of the BoNTs goes beyond this mechanism, taking into account a number of other factors, among them inhibition of pain neurotransmitter peptides such as substance P, enkephalin, calcitonin gene-related peptide (CGRP), and glutamate,15–17 anti-inflammatory effect,17 and suppression of muscle spindle discharge resulting in reduced excitation of gamma and alpha motor neurons.18 It has been shown that pain improves sooner and independently from neck posture in CD after BoNT treatment.19,20
Currently, three formulations of BoNT-A are commercially available for human use: Botox® (Allergan, Inc.), Dysport® (Ipsen Limited), and Xeomin® (Merz Pharmceutical). In the US, BoNT-B is distributed iunder the name Myobloc®, and in Europe under the name Neurobloc® (Solstice Neurosciences, Inc.). In the US, only Botox and Myobloc are approved by the Food and Drug Administration (FDA) and marketed for medical practice. Recently, the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology (AAN) reviewed the literature on BoNT treatment in CD and identified seven class I (blinded, prospective) studies.21 Six of these studies compared BoNTs with placebo and one compared BoNTs with trihexyphenidyl. The six included one with BoNT-A (Botox), two with BoNT-A (Dysport), and three with BoNT-B (Myobloc). All six studies depicted superiority of BoNTs to placebo in terms of efficacy and safety.22–27 The trihexyphenidyl study28 was conducted with BoNT-A (Dysport), which was proved to be more efficacious than tirhexyphenidyl in improving motor disability and movements while producing fewer adverse effects. The AAN subcommittee concluded that BoNTs are established as safe and effective for the treatment of CD, and that BoNT injections should be offered as a treatment option to patients with CD (level A). Improvement of pain and abnormal head and neck postures in these studies ranged from 60 to 95%. Comella’s recent review additionally analyzed four double-blind studies that compared two BoNTs with each other in terms of efficacy, tolerability, and side effects.29 Of these four studies, two compared BoNT-A (Botox) with BoNT-A (Dysport),30,31 one compared Botox with BoNT-A (Xeomin),32 and one compared Botox with BoNT-B (Myobloc).33 One of the two Dysport/Botox studies in CD showed comparable efficacy and side effects when the investigators used the ratio of three Dysport units to one Botox unit.30 In another Dysport/Botox study using two ratios of 3:1 and 4:1, patients who received Dysport had more improvement in terms of pain and neck posture, but showed more side effects.31 The Xeomin/Botox study32 showed no difference between the two in terms of improvement of total TWSTRS score and TWSTRS pain score. Both BoNTs displayed the same frequency of side effects in this study. A ratio of 1:1 was used for this study.
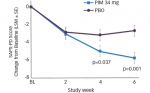
The comparison studies between Botox and Myobloc are more relevant to the practice of BoNT therapy in the US. In a blinded study, Comella et al.33 compared improvement of TWSTRS score at four weeks between 74 and 65 CD patients who received Botox and Myobloc, respectively. There was no significant difference between the two groups with regard to TWSTRS score. Rates of dysphagia and dry mouth were significantly higher in the Myobloc group (48 versus 19% and 80 versus 41%, respectively). The higher incidence of these adverse effects may be due to the structured interview adverse effect assessment utilized in the study, and also to the fact that all patients were naïve to Myobloc. The duration of Botox action was marginally longer than that of Myobloc (14 versus 12.1 weeks). A recent European double-blind, multicenter study34 compared Botox with Myobloc in naïve patients with CD (55 Botox, 56 Myobloc). The study showed no significant difference between the two in terms of TWSTRS total score or subscores (pain, severity, or disability) at four weeks, or in terms of duration of action. There was also no statistically significant difference between the two in terms of dysphagia, pain at the site of injection, and moderate or severe dryness of the mouth. Mild dryness of the mouth was more common in the Myobloc group.
Emergence of unresponsiveness after chronic use of BoNTs is a treatment obstacle. A comprehensive review of CD in 1998 cited a figure of 5–10% for unresponsiveness.6 Multiple factors could contribute to the clinical unresponsiveness, including the development of neutralizing antibodies. Although unresponsiveness often relates to the development of neutralizing antibodies, some patients continue to respond despite having them. The exact correlation between neutralizing antibodies and the clinical unresponsiveness is not well established or understood at this time.
Administration of large doses of BoNTs in one session (>300 units for Botox) and short intervals between the injections (<3 months) enhances antibody formation. In practice, unresponsiveness is rare with the new formulation of BoNT-A (Botox, introduced after 1997). Jankovic et al. found no neutralizing antibodies in a study of 119 patients treated with the new formulation, whereas 9.5% of the patients treated with the old formulation had the antibodies.35 Brin et al.36 studied the incidence of neutralizing antibodies in 326 patients with CD followed for an average period of 2.5 years with repeat Botox treatments (mean nine treatments). Four patients (1.2%) developed neutralizing antibodies and three of 251 patients (1.1%) who completed the study became unresponsive during treatment. These figures are considerably lower than the incidence of 5–10% cited in the earlier reports.6 The lower figures are probably due to the lower protein content of the new Botox preparation (5 versus 25ng) and better injector education using smaller dose/session, avoiding boosters, and adhering to longer injection intervals. The current literature supports the notion that, like BoNT-A (Botox), BoNT-B (Myobloc) also maintains its effectiveness over long-term use.37 However, immunoresistance to BoNT-B has not been well studied and warrants investigation.
Surgical Treatment
Deep brain stimulation (DBS) of globus pallidus pars interna (GPi) has shown promise in small prospective studies. A recent Canadian multicenter, double-blind study of 10 patients demonstrated 43% improvement of dystonia severity score and 59% improvement of TWSTRS total score compared with the pre-treatment values.38 A previous single-center study of 10 patients with refractory CD with long-term follow up (31±21 months) also reported 55% improvement of dystonia severity score after DBS.39 These reports are encouraging, since the results are higher than the 20–30% improvement reported for selective rizotomy with myotomy.40,41 Furthermore, DBS may have less permanent side effects than rhizotomy, and patients with rizotomy may require repeat operations for re-innervation of the target muscles.40
Cervical Anatomy and Injection Technique
The human neck contains many muscles, but only a handful are actively involved in CD. The easy surface accessibility of these muscles for injection accounts for the high rate of successful BoNT therapy of CD. Table 1 shows the function of the common contributing muscles to the symptoms of CD and the dose range of BoNTs for each muscle.
BoNT treatment should be performed by a clinician with in-depth knowledge of the neck muscles and their nerve supplies. Those new to the treatment should attend workshops and seminars that provide the relevant literature on BoNT therapy and offer opportunities to learn the intricacies of the injection technique from an experienced injector. The injector needs to be familiar with the types of toxin in use, their differences, their methods of dilution, and their side effects. If the BoNT is prepared by someone other than the injector, the injector needs to check and re-check proper dilution in order to avoid overdosing small muscles, which may result in excessive weakness. The injection session begins by informing the patient about the possible side effects of BoNT therapy. The injector then observes the position of the head and neck for a few minutes. Some patients need to be told not to try to suppress the neck deviation so the observer can fully appreciate the abnormal head and neck position. In case of torticollis, the most common form of deviation, the patient is asked to turn the head slowly and fully first to one and then to the other side so that the injector can see the side of movement limitation. In many patients, moving the head toward the side of limitation produces head jerks. In many patients with torticollis, a simple injection scheme that encompasses contralateral sternocleidomastoid and ipsilateral splenius group muscles can produce significant improvement of pain and range of head motion. In fact, it is wise to start the injections with a simple scheme and a modest dose. The injector can refine the technique with subsequent treatments. In CD, injections can be performed without electromyogram (EMG) since most affected muscles are anatomically well defined and easily accessible. Many injectors prefer using EMG and believe the added precision is necessary. At this time, the Therapeutics and Technology Assessment Subcommittee of the AAN considers the role of electromyography “not established“ in CD.21
Conclusion
Intramuscular administration of BoNTs is the most effective mode of treatment in cervical dystonia. In trained hands, the treatment is a short procedure and has a safe side effect profile. The literature indicates that types A and B are comparable in terms of their efficacy, but the units are not interchangeable and need to be individualized. Serotypes A and B are also comparable in terms of rare serious side effects (moderate or marked dysphagia or unwanted weakness). Mild dry mouth is more commonly seen with type B treatment. One type of serotype A (Xeomin) does not have complexing proteins. Whether this translates into a low rate of clinical responsiveness is yet to be determined. Neutralizing antibodies and unresponsiveness are rare with the current formulation of type A (Botox). Unresponsiveness and neutralizing antibody formation need to be further studied with both type A (Dysport) and type B (Myobloc). The correlation between neutralizing antibodies and clinical unresponsiveness is not well established and requires further study. In patients who do not respond to BoNTs, DBS or selective rhizotomy are the available options. ■