Proceedings of a symposium presented at the 13th European Congress of Epileptology, Vienna, 26th August 2018, sponsored by LivaNova.
The management of patients with drug-resistant epilepsy (DRE) or treatment-resistant depression (TRD) remain serious challenges. Both conditions are common; epilepsy is believed to affect 0.5–1.0% of the global population, of whom, 20.0–30.0% are said to be drug-resistant.1,2 Depression was reported to affect 6.9% of the European population in one large study (12-month prevalence).3 Among depressed patients, 12–20% are treatment-resistant which, in the US, is estimated to increase the societal cost of the disease by $29–48 billion/year.4 Both these conditions therefore, exert a serious burden on substantial numbers of people, their families and healthcare providers. Depression is known to be the most common comorbidity in epilepsy with up to 62% of patients with DRE displaying depressive symptoms.5 This may also be reflected in the 22% higher suicide rate in patients with epilepsy compared with the general population.6
Among the currently available treatment options for DRE, resective surgery is the only curative treatment.7–10 Temporal lobe resections in optimal patients produce 60–70% seizure freedom after 2–5 years compared with 0–10% with medical therapy only.11,12 However, surgery is not suitable for a large number of patients with DRE and many are not referred to tertiary centres that can provide non-pharmacological treatments. In addition, practicing neurologists tend to have cautious attitudes to surgery and are often uncertain about eligibility criteria.13,14
For patients with DRE for whom surgery is not appropriate, neurostimulation may be an option to reduce seizure burden.15 Neurostimulation therapies have been under continuous development since the 1950s and involve the implantation of devices that deliver electric current or a magnetic field to neural tissues to modulate neuronal activity and treat psychiatric disease, spasticity and pain.
Table 1: Clinical effects of three different neurostimulation methods for the treatment of resistant epilepsy
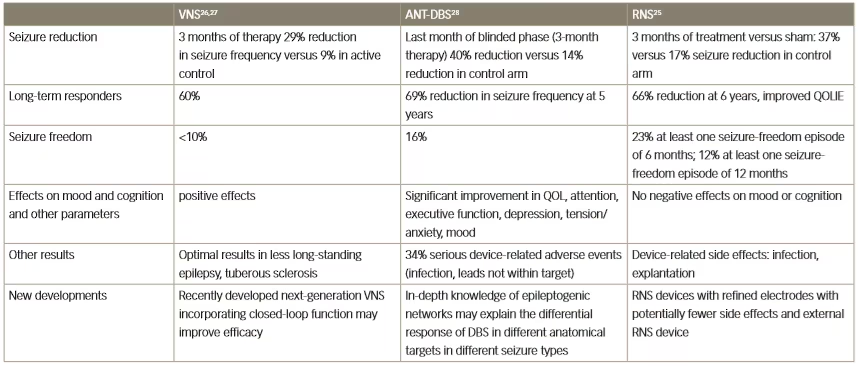
ANT-DBS = anterior thalamic deep brain stimulation; QOL = quality of life; QOLIE = Quality Of Life In Epilepsy Inventory; RNS = responsive neurostimulation;
VNS = vagus nerve stimulation.
Neurostimulation therapy is an emerging approach to various neurological disorders, especially Parkinson’s disease and epilepsy.16,17 In epilepsy, neurostimulation can be applied at different points using extracranial or intracranial approaches. These therapies also include open-loop deep brain stimulation (DBS), in which an intermittently stimulating electrode is inserted into the anterior thalamus. They also include closed-loop vagus nerve stimulation (VNS), in which an electrode sends electrical signals to the brain via the vagus nerve in the neck in an intermittent pattern but also in response to seizure-associated changes of heart-rate.18
In addition to managing epilepsy, neurostimulation also provides a valuable adjunctive treatment for the management of TRD. Depression is a highly common and serious comorbidity in epilepsy and is a predictor of poor outcomes such as drug resistance.5 This is demonstrated by studies showing that the prevalence of depressive symptoms in well-controlled epilepsy is approximately 33% whereas in drug-resistant disease it is 62%.19 In general populations, major depressive disorder is often recurrent (60% experience a recurrence within 5 years)20 and 20–35% of patients who experience an episode develop chronic depression.21–23 The difficulty of treating TRD was highlighted in the large-scale STAR*D (Sequenced Treatment Alternatives to Relieve Depression) trial (n=4,041) which investigated treatment efficacy in major depressive disorder. In this study, medication resistance was shown to predict relapse and there was a declining likelihood of remission with increasing lines of treatment.24 There is, therefore, a serious unmet clinical need for effective treatments for better long-term management of both DRE and depression, the most common comorbidity in epilepsy.
A prestimulation evaluation protocol for patients with drug-resistant epilepsy
Several different modalities of neuromodulation are currently in clinical use or are in development for use in various neurological disorders. The three principal types are: VNS, anterior thalamic-DBS (ANT-DBS) and responsive neurostimulation.25–28 Key clinical trial results for these three types in epilepsy are summarised in Table 1. The efficacy of these methods is broadly similar; VNS has achieved a long-term responder rate of 60% whereas with ANT-DBS there was a 69% reduction in seizure frequency at 5 years of treatment, and with responsive neurostimulation there was a 66% reduction in seizure frequency after 6 years of treatment.25–28
The various types of neurostimulation modalities currently available, or in development, target epileptogenic networks in different ways.7,29–31 This raises the question: which type of neurostimulation for which patient? Long-term efficacy appears to be similar with VNS, ANT-DBS and responsive neurostimulation, although seizure freedom may be higher with ANT-DBS and responsive neurostimulation. However, ANT-DBS and responsive neurostimulation have been used for focal epilepsy only whereas the VNS trials included patients with generalised epilepsy syndromes who are very difficult to treat successfully. This difference in populations studied and many other factors, limits comparability of these modalities.
The invasiveness of neurostimulation treatments is variable, which might affect choice, but there have been no direct comparative trials to help choose between them. To address this issue, a prestimulation protocol should be designed which comprises a series of rationally chosen investigations that evaluate the presence of signs and biomarkers for response to various neurostimulation therapies.32 These signs/biomarkers should reflect the susceptibility of the individual’s epileptic network to a given neurostimulation technique, as summarised in Figure 1. A currently applied strategy for choosing between the different stimulation therapies is based on the availability of the different therapies and the indications for which they are approved in geographic regions. It is clear that VNS is a more versatile therapy than DBS and responsive neurostimulation and is suitable for generalised, unifocal, bifocal and multifocal epilepsy. Furthermore, VNS also has benefits in treating comorbid depression, unlike DBS, and it is not associated with a risk of memory loss and is less invasive than DBS. In addition, VNS is also suitable for the treatment of both adults and children.
A true prestimulation protocol, as outlined in Figure 2, aims to identify responders. To do this, it is necessary to determine biomarkers that are predictive of a response to each treatment; however, at present, there is a lack of understanding of the mechanisms of action of neurostimulation on different brain parameters. These mechanisms are likely to be entirely different since non-responders to one type of neurostimulation may respond to another. In addition, efficacy may be improved using combinations of therapies. It is also likely that the mechanisms of action of neurosurgery are completely different to that of neurostimulation so the biomarkers required to identify suitable patients are also likely to be different.
Figure 1: Available facts that inform the choice of neurostimulation modality in epilepsy treatment
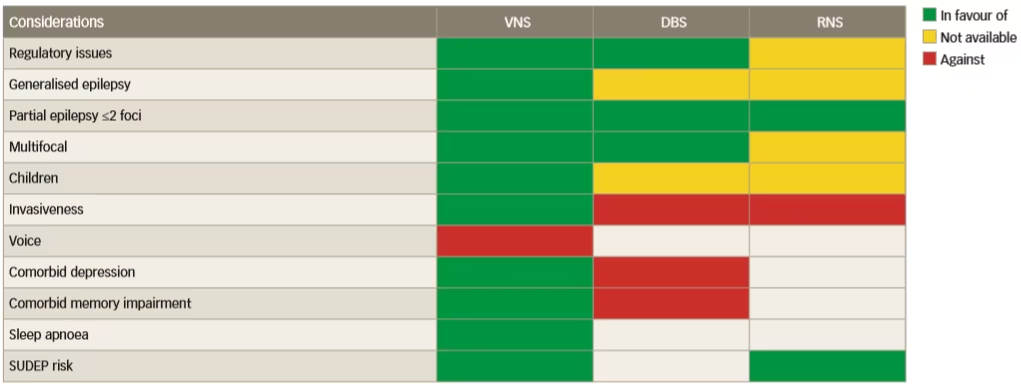
DBS = deep brain stimulation; RNS = responsive neurostimulation; SUDEP = sudden unexpected death in epilepsy; VNS = vagus nerve stimulation.
Reused with permission from Carrette et al. 2016.29
Figure 2: Proposed design of a prestimulation evaluation protocol
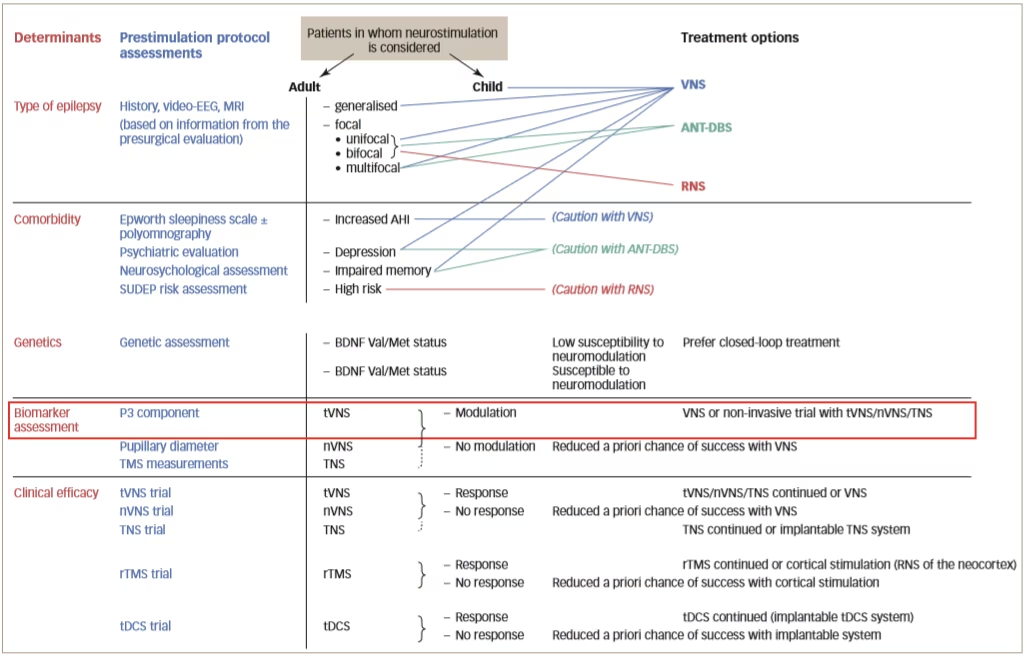
AHI = apnoea-hypopnea index; ANT-DBS = deep brain stimulation of the anterior thalamic nucleus; BDNF = brain derived neurotrophic factor; EEG = electroencephalography; MRI = magnetic resonance imaging; nVNS = non-invasive vagus nerve stimulation; RNS = responsive neurostimulation system; rTMS = repetitive transcranial magnetic stimulation; SUDEP = sudden unexpected death in epilepsy; tDCS = transcranial direct current stimulation; TNS = trigeminal nerve stimulation; tVNS = transcutaneous vagus nerve stimulation; Val/Met = valine/methionine; VNS = vagus nerve stimulation.
Reused with permission from Carrette et al. 2017.32
One important pathway into the brain for neurostimulation is via the vagus nerve and the locus coeruleus, which is a notable source of noradrenalin release into the brain.33 In rat models, if the vagus nerve is stimulated, noradrenalin is increased in the hippocampus.34 Furthermore, if lesions are created in the locus coeruleus, seizure activity is induced or worsened.35 In another rat model, pilocarpine infusion into the brain was used to induce epileptic seizures but these could be reduced with VNS.36 This effect was most marked in rats showing the largest increase in noradrenalin. These findings were subsequently replicated in dogs.37
In patients, a marker of noradrenalin in the brain is the event-related potential P300 which can be monitored in a non-invasive way using electroencephalogram (EEG) electrodes applied to the scalp.38 In one study, among 10 epileptic patients who responded to VNS, there was a significant increase in P300 amplitude during stimulation whereas in 10 non-responders no such increase was observed.39 This is good evidence that neurostimulation affects noradrenalin activity which reduces seizure frequency and/or intensity. Prospective clinical studies are in progress using other types of neurostimulation such as transcutaneous VNS to further investigate this response and help identify patients who will respond to VNS treatment.
In the treatment of epilepsy, therefore, selection of different neurostimulation modalities is currently driven by availability of the treatment at different locations, and comorbidities. The mechanisms of action of neurostimulation, however, are not fully understood. Predictors of response need to be further elucidated to enable the development of an evidence-based prestimulation protocol that would take account of electro-kinetics and dynamics and the electrical field–nervous tissue interface. Animal models and clinical evidence suggests that the modulation of noradrenalin and its transmission system are important components of the mechanism of VNS in reducing seizures.34–37 To monitor this, P300 may be an effective non-invasive method of measuring changes in the brains of patients. However, the value of P300 as a predictor of response needs much further investigation.
Desyncing the cortex – investigating vagus nerve stimulation mechanisms with a quantitative electroencephalogram method
VNS with implanted electrodes to control epileptic seizures was first used in 1988;40 the treatment was approved for use in Europe in 1994 and by the US Food and Drug Administration in 1997. Despite its long duration in clinical use, the mode of action of VNS is not fully elucidated and there is a lack of reliable biomarkers that are predictive of a response to the treatment. Some intriguing observations, however, suggest that VNS impacts brain EEG synchrony and the extent of this effect could be linked to the therapeutic effect.41–44
VNS modifies cortical activity via changes in synchrony in several brain regions including the nucleus of the solitary tract, the parabrachial nucleus, the thalamus, the amygdala, insular cortex, and the orbitofrontal and cingulate cortices.45 Furthermore, various positron emission tomography (PET), functional magnetic resonance imaging (fMRI) and single photon emission computed tomography (SPECT) studies have shown that VNS causes metabolic changes in multiple brain regions.46–49 In one study, 27 patients with refractory epilepsy were monitored using (99 m)Tc-ECD (ethyl cysteine dimer) SPECT.49 Following VNS treatment, significant changes in regional cerebral blood flow were found in the thalamus, the hippocampus and the para-hippocampal gyrus. In addition, acute limbic hyper-perfusion and chronic thalamic hypo-perfusion were shown to correlate with positive clinical efficacy.
Classical animal model studies conducted during the 1950s and 1960s showed that VNS affected acute epileptic phenomena such as slow waves and spiking. Desynchronisation could be observed, especially with higher amplitude or frequency of stimulation.50,51 This early work also showed that the induction of EEG spiking using strychnine could be suppressed by VNS-induced desynchronisation. Increasing the voltage of the stimulus from 0.40 V to 0.55 V and the duration from 0.1 milliseconds to 0.7 milliseconds resulted in desynchronisation.50,51 The authors subsequently concluded that the vagal afferent system comprises multiple fibre groups which are able to initiate and maintain either synchronisation or desynchronisation of the EEG.52 Investigations on EEG in humans have also shown that VNS could impact brain EEG synchrony but also functional connectivity, a concept which describes the links between signals recorded from distinct brain regions.41–44
Functional connectivity links have been studied using mathematical and statistical relationships between signals such as spikes, EEG, magnetoencephalography and fMRI. Functional connectivity does not relate to direct anatomical connections but underlies physiological functions, and monitoring its alteration is a means of studying the pathophysiology of brain disease.53–55 Functional connectivity plays an important role in epileptic seizures; there is a large increase in EEG synchronicity during the development and onset of a seizure which peaks before termination. A study has shown that during a seizure there is a large increase in connectivity within epileptogenic networks which subsequently decreases after termination (Figure 3).56 Another study showed that during absence seizures in children there was an increase of EEG synchronisation in all frequency bands, and functional network topology became more ordered compared with pre-ictal patterns.57
One of the most important clinical manifestations of functional connectivity alteration in epileptic seizures is loss of consciousness. Consciousness has two key components, vigilance/wakefulness and awareness.58 Studies show that sudden alterations in consciousness in seizures are accompanied by non-linear increases of neural synchrony within distant cortico-cortical and cortico-thalamic networks.59 Tonic/clonic seizures are characterised by low vigilance with low awareness whereas absence or complex partial seizures show higher vigilance with low awareness. The brain regions controlling these aspects are separate but increased knowledge of their function and complex interactions is needed to better understand conscious and vegetative states and the effects of seizure.60,61
Various small clinical studies using intracranial or intracerebral EEG have shown interictal changes in functional connectivity in the separate propagation and epileptogenic zone networks within the brains of patients with epilepsy but decreased functional connectivity in non-involved zones.62–65 Some other studies have investigated interictal changes in functional connectivity produced by VNS. An example is one that investigated intercortical synchronicity during VNS ON and OFF periods using EEG in 19 adult patients with DRE.42 The phase lag index, which is a measure of synchronicity determined at all scalp electrodes, was generally lower in VNS responders than non-responders. There was also a lower global level of resting state synchronisation in responders than in non-responders during both ON and OFF periods (p<0.0001). A further study used stereotactic EEG to investigate functional connectivity in five patients with epilepsy who were undergoing VNS therapy.41 Non-linear regression analysis of stereotactic EEG results revealed no change or higher values (indicating higher functional connectivity) for four VNS non-responding patients during ON periods than OFF periods but lower values for one patient who was a responder (Table 2 and Figure 4). These results suggested that the mechanism producing a response to VNS involves the induction of a lower functional connectivity.
Figure 3: Characterisation of epileptogenic networks in the temporal lobe during the transition from pre-ictal to seizure activity and termination
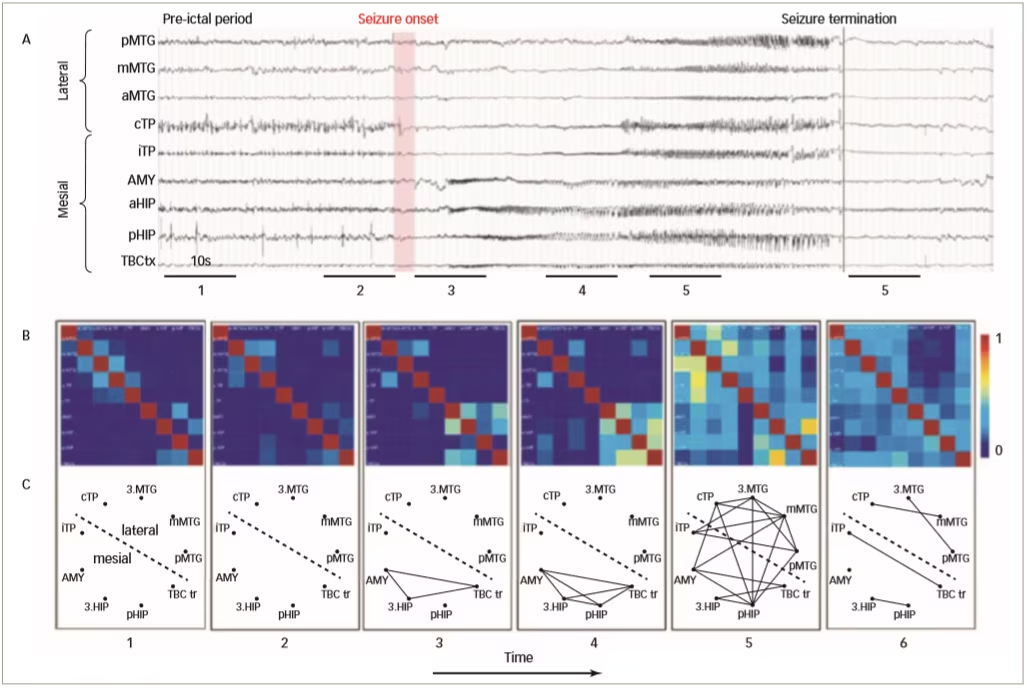
- EEG recordings from a patient with temporal lobe epilepsy; B. colour-coded non-linear correlation matrices obtained from the pairwise computation of nonlinear correlation coefficient h2 over six different 10-second intervals chosen during the pre-ictal period (1, 2), the ictal period (3, 4, 5) and after seizure termination; C. Graphical representation in which the lines indicate ‘abnormally strong’ couplings between the two considered structures (graph nodes).
AMY = amygdala; EEG = electroencephalogram; HIP = parahippocampal gyrus; MTG = middle temporal gyrus; TBC = temporobasal cortex; TP = total power
Reused with permission from Wendling et al. 2009.56
Table 2: Synchronisation patterns in five epileptic patients (four non-responders and one responder)
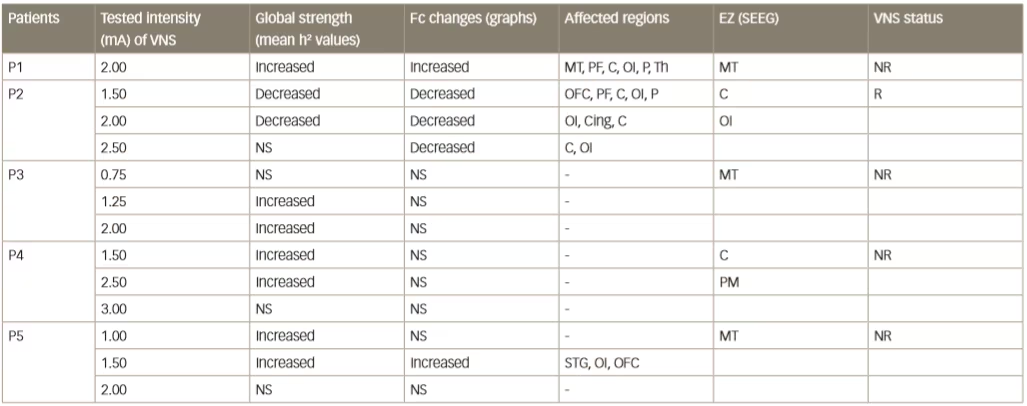
C = central cortex; Cing = cingulate gyrus; EZ = epileptogenic zone; Fc = functional connectivity; MT = mesial temporal region; NR = non-responder; NS = no significant change; OFC = orbitofrontal cortex; OI = operculo-insular; P = parietal cortex; PF = prefrontal cortex; PM = premotor; R = responder; SEEG = stereoelectroencephalography; STG = superior temporal gyrus; Th = thalamus. Reused with permission from Bartolomei et al. 201641
Figure 4: Synchronisation patterns in five epileptic patients (four non-responders and one responder)
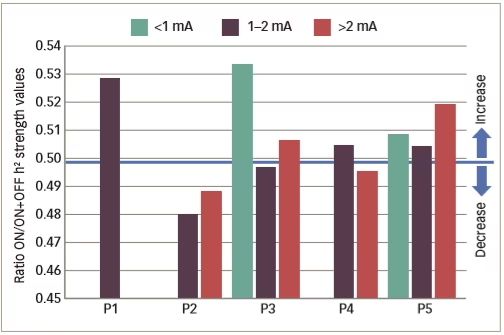
P1–5 = patient 1–5 . Reused with permission from Bartolomei et al. 2016.41
In a further clinical study of VNS in epilepsy, 51 patients with epilepsy were implanted with VNS devices (AspireSR® VNS Therapy System, Houston Texas, USA) and EEG data were collected before, and 1 month after, VNS treatment.44 Data from 16 patients (103 pre-VNS and 102 post-VNS seizures) show that only responding patients had decreased connectivity and spatial synchronisation (Figure 5). Automated delivery of VNS therapy reduced ictal spatial synchronisation in responding patients with a ≥50% reduction in seizure frequency; this finding has potential as a predictor of long-term response to VNS therapy.
Overall, clinical studies show that DRE produces brain regional changes in functional connectivity which can be observed as EEG synchrony. VNS therapy during the interictal state may change functional connectivity patterns; responding patients show lower level of synchronicity. In one study that used the AspireSR® VNS system, stimulation during a seizure decreased EEG synchrony but only in responders.44 The links between VNS parameters and functional connectivity/synchronicity are, as yet, unknown and may have a non-linear relationship. Further studies are therefore needed to investigate the predictive value of functional connectivity/synchrony findings on VNS response.
Learning from depression – insights from functional imaging of neurostimulation
In addition to treating epilepsy, VNS has shown promising clinical results in various studies on the antidepressant treatment of TRD. The mechanism of this effect is not currently fully understood; however, the afferent neuroanatomical pathways of the vagus nerve provide some insights. The afferent vagal fibres enter the brainstem at the level of the medulla. Here, most of the afferent projections travel toward the brain via the nucleus tractus solitarius, synapsing in the parabrachial nucleus. Vagal afferents from the parabrachial nucleus project to multiple cerebral regions such as limbic structures (amygdala and insular cortex), autonomic structures (hypothalamus and periaqueductal gray), and reticular structures (thalamus), all of which are known to be associated with mood regulation.66
A pivotal trial of VNS in TRD (n=221) demonstrated that the treatment effect on depression was gradual.67 The proportion of patients who responded (as defined by a ≥50% reduction in depressive symptoms from baseline using standard depression scales) at 3 months was only 14–17%. However, at 24 months, the response rate had increased to 27–32%. The improvements in depression scores were also sustained in most patients for 1–2 years or longer.67 The largest and longest real-world study of VNS in TRD, was a prospective, open-label registry study involving 795 patients with major depressive episodes.68 This study compared adjunctive VNS versus treatment as usual (any antidepressant treatment available to the patient, including ECT). Patients were required to have had a major depressive episode of at least 2 years’ duration or three or more depressive episodes, and failed four or more depression treatments. Patients receiving adjunctive VNS showed significantly higher 5-year cumulative antidepressant response rates than those receiving usual treatment (67.6% versus 40.9%, p<0.001) and a significantly higher remission rate (43.3% versus 25.7%, p<0.001). There were also significant differences with adjunctive VNS over usual treatment in 5-year cumulative response rates in the subgroups of patients who were ECT responders and ECT non-responders (p<0.001 in both cases).66 This is further substantiated other studies, which have demonstrated the powerful antidepressant benefits of VNS in TRD.67,68
The effects of VNS in depression have also been investigated in various neuroimaging studies. In one such study, four patients with TRD were intravenously given radio isotope-labelled water as a tracer (traces real-time cerebral blood flow changes).69 Comparing PET scans taken before and after VNS revealed that the treatment substantially increased cerebral blood flow in the right anterior insula, the left orbitofrontal, left inferior putamen and left and right anterior cingulate region. Decreases were found in the bilateral temporal cortex and right parietal area. These regions showing changed flow were consistent with brain structures associated with depression and the afferent pathways of the vagus nerve. Similarly, an fMRI study of 10 patients with TRD investigated the chronic effect of VNS on TRD by monitoring oxygen uptake in the right anterior insula, which is indicative of increased activity in response to VNS treatment.70 There was a significant linear correlation between changes in right insular activity and duration of VNS therapy (r2=0.286, p=0.0001). Initially there was an increase in right insular activity; however, over time this decreased and at 30 weeks an ‘inflection point’ was reached, after which there was an overall reduction in activity. Furthermore, there was a significant linear correlation between right insular activity and depression score (Hamilton Depression Rating Scale [HRDS]). These findings supported previous observations indicating that the effect of VNS on the depressed brain is most likely gradual and represents an adaptation occurring in regions known to be associated with mood (in this case insular cortex).
Subacute and chronic changes associated with VNS treatment of TRD have also been investigated using [18F] fluodeoxyglucose PET (FDG-PET). In a study of 13 patients with TRD, FDG-PET and fMRI scans were taken before the VNS devices were turned on and again at 3 and 12 months after starting treatment.71 After 3 months, only 2/13 patients showed an antidepressant response or remission (reduction in the 24-item HRDS). However, at 12 months, 9/13 showed a response or remission. At 3 months, FDG-PET scans showed a right-sided deactivation pattern in responders but not in non-responders (decreased mean regional glucose uptake). However, at 12 months, the FDG-PET scans in the responders no longer demonstrated right cortical decreased regional mean glucose uptake, but rather, a return to baseline activity. Notably, however, there was an increase in mean glucose uptake in the midbrain tegmental region. These changes were not observed in the VNS non-responders. A closer sub-analysis revealed changes in the ventral tegmental area (VTA) in the VNS responders, a region known to be critical in dopaminergic function in the brain.71 This analysis of quantitative results in the VTA region demonstrated a significant positive (increasing) linear trend for VNS responders (p=0.002), a negative (decreasing but non-significant) linear tread for VNS non-responders (p=0.15) and a significant overall linear group x time interaction (p=0.001).71 The physiological reason for these differences between responders and non-responders is unknown but other studies suggest it could involve VNS impacting the suppressive dopaminergic effects of the habenula on the VTA.72
Figure 5: Change in electroencephalogram synchronisation from pre- to post-vagus nerve stimulation in three responding patients with epileptic seizures
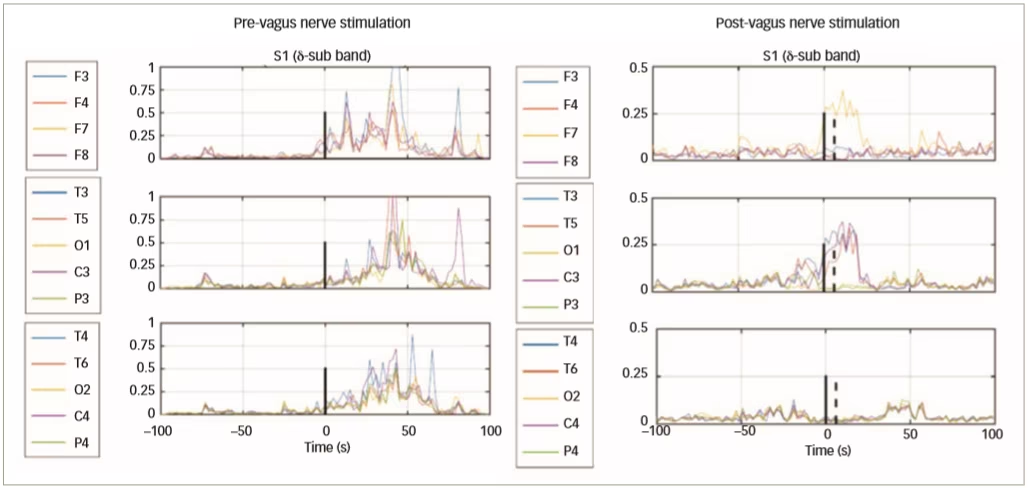
Reused with permission from Ravan 2017.44
Following positive outcomes of VNS treatment of TRD, some studies have evaluated the use of neuroimaging to predict antidepressant outcomes. In a recent study that included 12 patients, four brain regions were chosen for FDG-PET imaging based on their known anatomical association with vagus nerve projections and evidence supporting their involvement in depression.73 These areas included the orbitofrontal cortex, the anterior cingulate, the anterior insular cortex, and the dorsolateral prefrontal cortex. VNS responders showed increased baseline metabolic activity in the orbitofrontal cortex, whereas non-responders showed increased baseline metabolic activity in the insular cortex. A combined analysis of these results showed a correlation between baseline orbitofrontal cortex/insular cortex activity and change in HRDS-24 item scores (r2=0.005, p=0.005). In this study, therefore, baseline orbitofrontal cortex/insular cortex activity appeared to be predictive of VNS treatment response.
The collected evidence from functional imaging shows that the efficacy of VNS in depression is likely to act via afferent pathways that project to brain regions including the locus coeruleus, dorsal raphe nucleus amygdala, insula, striatum, and orbitofrontal cortex that are responsible for the maintenance of mood. In the clinical studies discussed, the response to VNS in TRD is gradual; significant clinical responses in many patients do not emerge until 6–12 months after starting treatment. Imaging studies using fMRI and FDG-PET show that these delayed effects of VNS could be the result of an adaptive response in the brain.70,71,73 FDG-PET imaging also shows different areas of activity in the brain in VNS responders compared with non-responders; these patterns changed over a 12-month period. Imaging also shows increased metabolism in the mesencephalic VTA region in responders compared with lower metabolism in non-responders. Furthermore, it may be possible to predict a response to VNS in TRD using FDG-PET since responders show greater pre-treatment activation of orbitofrontal cortex, whereas non-responders show increased activation of insular cortex.
Conclusion
Available evidence indicates that neurostimulation can be highly effective in the long-term management of both DRE and TRD in many patients. These are serious conditions that place a substantial burden on health and healthcare resources worldwide and are responsible for considerable morbidity and mortality.1–4 At present, the choice of neurostimulation methods is expanding but the parameters for their use are not well defined.32 There is a lack of comparative trials or indeed, reliable biomarkers indicating which patients are likely to respond. For DRE, the proposed prestimulation protocol has the potential to help decide which of the many different neurostimulation modalities are suitable for which patients and to guide best practice.32 However, it is currently based primarily on comorbidities and availability, and more investigation and data are needed to inform this approach.
Among neurostimulation methods, VNS has shown marked efficacy in DRE.32 This appears to be a result of the vagus nerve afferent pathways into the brain stimulating various regions that are involved with noradrenalin signalling. The monitoring of such activity using P300 as a marker has proven to be a valuable means of studying VNS effects.38 However, more work is needed to determine the effect of increasing VNS amplitude and P300 response. EEG studies have shown that in epilepsy, there is a higher degree of synchronisation between certain neural networks, and that VNS can result in desynchronisation which may diminish or terminate seizures.50–52 This treatment has also been shown to affect functional connectivity between distinct brain regions.53-55 These results are supported by imaging studies using fMRI, PET and SPECT which detected changes in blood flow in separate areas such as the thalamus and hippocampus following VNS treatment.46-49 Changes in neuronal synchrony during epileptic seizures have also been shown to affect different components of consciousness and different areas are involved in tonic/clonic versus absence seizures.59-61 Whilst VNS is clearly effective, its precise mechanism on functional connectivity, however, is not yet understood.41,44 It is also unclear which anti-epileptic drugs are most suitable to combine with neurostimulation to augment the effects and provide the best long-term control.
The effects of VNS on TRD are also likely to result from the anatomy of the vagus nerve and the brain regions it projects to that are directly involved in controlling mood.66 Various studies indicate that the anti-depressive effect is gradual; many patients show little or no response before 6 months.67,68 During this time, the brain appears to undergo an adaptive change in response to long-term VNS treatment. At the same time, FDG-PET studies show inverted metabolism in areas including the VTA, orbitofrontal cortex and anterior insular cortex following VNS treatment.69 It is notable that VNS increases or decreases metabolism in different brain areas in responders versus non-responders. These findings may provide a simple means of identifying patients with TRD who are suitable for VNS and those who are not. The reasons for these different responses to VNS in different patients, however, remain to be determined.
Neurostimulation, particularly VNS, is an increasingly valuable approach to the management of DRE and TRD. In the future, it is likely that increased understanding of its mechanism of action will better guide its use in these diseases, aid the development of improved techniques and enable the establishment of consensus treatment protocols.32 It may also lead to more efficient identification of patients who are suitable for neurostimulation and encourage greater use of the approach and to improve outcomes in a wider population of refractory patients who are currently very difficult to manage. ⬛













