Sleep-disordered Breathing
SDB can be grouped into two major subtypes: obstructive sleep apnea (OSA) and central sleep apnea.
Obstructive Sleep Apnea
Epidemiology
OSA is a highly prevalent disorder affecting both children and adults. It has been estimated that among the working population between 30 and 60 years of age, 2% of women and 4% of men meet the minimal diagnostic criteria for OSA.1 However, this number may be an underestimate as a subsequent investigation using polysomnography (PSG) showed that up to 93% of middle-aged women and 82% of men with moderate to severe OSA have not been clinically diagnosed by their physicians.2 OSA is approximately two to four times more prevalent in men than in women,3–5 and becomes more common with advancing age, occurring in 21–26% of men over 65 years of age.6 Independent risk factors for OSA include age, male sex, increased body mass index (BMI), hyperlipidemia, and alcohol ingestion.5,7 The influence of race and ethnicity is inconclusive. While some studies show no effect,5,8 others have suggested increased risk in African-American, American-Indian, and Asian populations, primarily based on weight and craniofacial dimensions.9–12 OSA may also be influenced by hormonal factors in women, with increased risk after menopause.4 The estimated prevalence of sleep-disordered breathing in children eight to 11 years of age is 2.2%, with increased risk among pre-term children9 and increased risk in boys compared with girls.8
Diagnostic Criteria
Patients with OSA typically experience sleep symptoms of snoring, nocturnal dyspnea, witnessed apneas, and nocturnal restlessness. These patients may also have difficulties with nocturia, diaphoresis, acid reflux, and drooling. During the day, many complain of excessive sleepiness, headaches, poor concentration, decreased energy, and depression.13,14 The diagnosis of OSA is based on a combination of clinical and laboratory data. Important components of the medical history include sleepiness severity as quantified by the Epworth Sleepiness Scale (an eight-point questionnaire designed to measure daytime sleepiness). Comorbidities such as hypertension and cardiovascular disease should also be evaluated. The physical examination includes measurement of BMI and neck circumference. The Mallampati classification can be helpful in predicting the severity of OSA by evaluating the anatomy of the oral cavity (see Figure 1).14
Scoring is as follows:
Class 1: Full visibility of tonsils, uvula, and soft palate.
Class 2: Visibility of hard and soft palate, upper portion of tonsils, and uvula.
Class 3: Soft and hard palate and base of the uvula are visible.
Class 4: Only hard palate visible.
Modified after Mallampati SR, Gatt SP, Gugino LD, et al., A clinical sign to predict difficult tracheal intubation: a prospective study, Can Anaesth Soc J, 1985;32:429–34.
Patients with findings suggestive of OSA should undergo confirmatory testing with nocturnal PSG. Typically carried out in a sleep laboratory, this test combines electroencephalography (EEG) to assess sleep architecture, staging an electro-oculogram to measure eye movements, surface electromyogram (EMG) to monitor body movements and respiratory effort, pulse oximetry to check for oxygen desaturations, electrocardiogram (EKG), and monitors for snoring, airflow, and nasal pressure.
OSA patients will have apneic episodes on PSG in the presence of respiratory muscle effort. An example of sleep apnea on the PSG is demonstrated in Figure 2. Clinically, significant sleep apnea is defined as absence of airflow for at least 10 seconds with an associated transient drop in oxygen saturation.14,15 Patients can also exhibit hypopnea (decrement in effort) without frank apnea. While precise criteria vary, hypopnea can be defined as a 30–50% reduction in thoracoabdominal movement for ≥10 seconds, with at least a 4% drop in oxygen saturation.11,16 Frequently, apneas and hypopneas result in arousals from sleep causing insomnia and fatigue.14
Diagnostic criteria for OSA include the apnea–hypopnea index (AHI), which measures the total number of apneas and hypopneas per hour of sleep. To fulfill the criteria for OSA, at least five apneas or hypopneas per sleep-hour (AHI of 5) are required, with associated evidence of increased respiratory efforts during the respiratory event, along with either complaints of hypersomnia, gasping, or choking during sleep, or witnessed loud snoring or breathing interruptions during the patient’s sleep.14,15,17 OSA can be further classified into mild (AHI of 5–15), moderate (AHI of 15–50), and severe (AHI of >50).15 Severity is influenced by various criteria, including the degree of hypoxia, the level of daytime sleepiness, and the presence of apnea-associated heart-rate variability.14
Pathophysiology
OSA and hypopneas are associated with pharyngeal obstruction during sleep, due in part to changes in oropharyngeal size and structure, resulting in intermittent airflow interruption and hypoxemia. Obesity can greatly contribute to this effect as adipose tissue deposition causes airway narrowing, reduced lung capacity, and impaired efficiency of gas exchange.17,18 Additionally, when obese patients sleep in the supine position, the diaphragm becomes displaced into a flatter and higher position, resulting in reduced inspiratory strength.19 Normal reductions in abdominal and intercostal muscle activity that occur during rapid eye movement (REM) sleep can further impair ventilation in OSA patients.20 Thus, subjects are more likely to have apneic events during REM sleep.20There is a strong association between habitual snoring and OSA.7 Numerous studies have demonstrated increased cardiovascular risk associated with snoring, including a large population-based trial in which men with snoring and daytime somnolence had double the 10-year mortality rate of normal controls.21 Snoring can result in pharyngeal trauma with edema and inflammation, subsequently narrowing the airway and possibly impairing the function of receptors responsible for maintaining airway patency in sleep.22,23 Adenotonsillar hypertrophy is a frequent cause of OSA in children and can often be surgically managed.24 In addition, maxillary and mandibular skeletal positioning can affect the size of the oropharyngeal cavity, and this has led to the development of OSA treatments with a variety of dental devices.24
Consequences
A diagnosis of OSA carries with it a significant degree of morbidity and mortality. There can be profound psychological consequences from excessive daytime sleepiness, including poor work and school performance, difficulty with complex motor tasks, sleepiness while driving, impaired attention and memory, and mood disturbances such as depression and anxiety.25–28 Approximately 50% of OSA patients also suffer from chronic morning headaches.14,25
Hypoxemia and hypercapnea during apneic events can have both immediate and long-term cardiovascular consequences. In the acute setting, repeated hypoventilation can cause a surge in sympathetic drive that raises blood pressure and heart rate.29 This predisposes to sudden death from myocardial ischemia and cardiac arrhythmias.30,31 In the long term, repeated bursts of sympathetic activity and hypertension result in increased rates of atherosclerosis, coronary artery disease, and strokes.32 Patients with a history of cardiac ischemic events who are treated for OSA have significantly lower rates of cardiac deaths compared with untreated OSA patients.29
Treatment
There is a range of treatment options for OSA that can be tailored to the severity of an individual’s symptoms (see Table 1). Behavioral modifications may be sufficient for mild disease, and these include weight loss, refraining from use of alcohol, tobacco, or sedatives, and avoidance of sleeping in the supine position.25 Interventions with intra-oral appliances such as mandibular advancement appliances can be particularly useful in snoring or mild disease.19,24 The current mainstay of therapy is nasal continuous positive airway pressure (CPAP), which can be used for all degrees of OSA, including in pediatric patients. Nightly CPAP use is effective in reducing daytime somnolence and improving oxygenation, sleep quality, and cardiovascular end points.25 Surgery for OSA that targets the tongue, palate, or hypopharynx may be an option for patients with severe disease or those who cannot tolerate CPAP.24 Tracheotomy is the treatment of last resort in patients with severe OSA if they also have significant cardiovascular comorbidities and are unable to tolerate CPAP. Currently, there are no effective pharmacological OSA treatments.33 Modafinil may be used to treat persistent sleepiness in patients who are compliant with CPAP and do not suffer from insufficient sleep.25 Other non-traditional treatments for OSA have been described that may improve symptoms by strengthening the upper airways, such as playing the didgeridoo.34
Central Sleep Apnea
Central sleep apnea is a distinct syndrome of dysrhythmic breathing that is often difficult to clinically distinguish from OSA. The syndrome is characterized by apneic episodes in sleep lasting at least 10 seconds accompanied by an absence of respiratory effort, in addition to an absence of airflow. Presenting symptoms and sequaelae of the disease can be similar to those of OSA, and in fact the two syndromes can co-exist. Central sleep apnea can occur either as a primary disorder of respiratory drive or secondary to a variety of medical conditions, including congestive heart failure, strokes, nasal obstruction, brainstem injury, or autonomic dysfunction.14,35 The pathophysiology may be due to derangements in the metabolic control system governing ventilation, which normally responds to systemic hypercapnia and hypoxia.35 Patients with central apnea have multiple arousals from sleep associated with deep breaths that lower carbon dioxide to a level that no longer stimulates ventilatory drive.18 This phenomenon can also be seen in Cheyne-Stokes respiration, where pulmonary edema and hypoxemia lead to hyperventilation and resultant hypocapnea in sleep, causing frequent apneas and sleep fragmentation.18 Central apneas are thought to occur primarily in sleep, because during wakefulness other stimuli such as vision and sound can provide input to compensate for impaired response to hypoxia or hypercapnia.35 A sustained pattern of central apnea most commonly occurs during non-REM sleep.18
Motor Disorders of Sleep
A variety of abnormal movements are associated with sleep or drowsiness. These can frequently result in sleep disruption among patients and their sleep partners.
Parasomnias
Parasomnias are defined as undesirable behaviors or experiences that are normally associated with wakefulness, which instead occur during sleep. They can be classified based on the sleep stage during which the majority of the events occur (transition between wake–sleep, REM sleep, or non-REM sleep).14
Rapid Eye Movement Sleep Behavior Disorder
Definition and Diagnostic Criteria
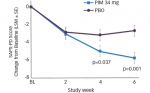
REM sleep behavior disorder (RBD) is characterized by the loss of muscle atonia that would normally occur in REM sleep, allowing for vigorous movements during dream sleep. Typically, RBD is associated with unusually violent or vivid dream content. Potentially injurious behaviors can arise in sleep, including punching, kicking, screaming, grasping, or running.36 The events last for seconds to minutes, but can sometimes occur numerous times during the night.37 Unlike nocturnal seizures, the movements seen in RBD are not stereotyped and tend to be complex and polymorphic.37 The diagnosis of RBD is based on a combination of historical features and PSG, which shows abnormally increased muscle tone on surface EMG leads during REM sleep, as shown in the example in Figure 3. Video monitoring should demonstrate that abnormal movements correspond with periods of excessive EMG signal.37
A 60-second period from the diagnostic polysomnogram (PSG) of a 77-year-old man who was referred to the sleep disorders clinic for evaluation of recurrent violent night-time awakenings. Illustrated in this figure is a typical spell that the patient was experiencing. He was noted to yell, jump from bed, and have complex body movements. The left anterior tibialis muscle as well as the chin electromyogram (EMG) both demonstrate abnormal augmentation of muscle tone, which, together with clinical history of dream enactment nocturnal behaviours, is highly suggestive of REM sleep behaviour disorder (RBD). Channels are as follows: electro-oculogram (left: LOC-A2, right: ROC-A1), chin EMG, electroencephalography (EEG) (left central, right central, left occipital, right occipital), two electrocardiogram (EKG) channels, left anterior tibialis EMG, snore channel, nasal–oral airflow, respiratory effort (thoracic, abdominal), and oxygen saturation (SaO2). Epidemiology
The prevalence of RBD is unknown, but it occurs more frequently in individuals >50 years of age and particularly in men.36 Approximately 60% of cases are idiopathic and 40% are associated with a neurological disorder such as narcolepsy or a synucleinopathy such as Parkinson’s disease (PD), Lewy body dementia (LBD), or multiple system atrophy (MSA).36 Recent findings have shown that RBD may be an early manifestation of parkinsonism, often preceding parkinsonian symptoms by many years.38 Patients with RBD can have other early features of PD, such as olfactory dysfunction and autonomic instability.36 Also, there are acute forms of RBD that are pharmacologically induced, usually secondary to use of a psychoactive medication or withdrawal from alcohol or barbiturates.39
The pathophysiology of RBD has not been precisely delineated, but multiple brain areas are thought to be involved, including the locus coeruleus in the pons, nuclei in the medial medulla, and striatal dopaminergic neurons.36 Untreated, RBD may result in physical trauma to the patient or his or her sleep partner, including bruises and fractures. Excessive daytime sleepiness is not usually a common complaint unless RBD is associated with narcolepsy.39 Treatment strategies include behavioral interventions, such as avoiding sleep deprivation and alcohol and removing dangerous objects from the bedroom. Pharmacological treatment is indicated when parasomnias occur frequently or result in physical injury.37 Benzodiazepines such as clonazepam 0.5–1mg at bedtime are the first-line treatment and are effective.37,39 Other medications include tricyclic antidepressants, dopamine agonists (pramipexole), and melatonin.39,40
Parasomnias During Non-rapid Eye Movement Sleep
Various parasomnias occur during slow-wave non-REM sleep, usually in the first part of the night. These include confusional arousals, somnambulism (sleep walking), and night terrors. Confusional arousals are commonly seen in children <5 years of age, but the prevalence decreases greatly by adulthood.14 They consist of arousals from sleep during which the patient is disoriented and exhibits inappropriate behavior for several minutes. Patients themselves rarely remember the events. It is thought to be due to a partial intrusion of wakefulness into non-REM sleep, and can be exacerbated by sleep deprivation, shift work, alcohol, or central nervous system depressant drugs.41 The episodes are self-limited, but efforts to interact with the patient should be avoided as they may cause confusion and lead to aggression.42 Confusional arousals usually abate with age and pharmacological treatment is rarely necessary. In severe cases, treatment may include benzodiazepines, serotonin re-uptake inhibitors, or tricyclic antidepressants.43
Somnambulism consists of walking and other complex motor behaviors that occur while the patient is still in slow-wave sleep with incomplete awakening on EEG.44 Risk factors include sleep deprivation, fever, use of psychoactive medications, or other medical conditions that disrupt slowwave sleep.44 The incidence ranges between 1 and 15% of the population, with the greatest prevalence seen among children between four and eight years of age.14
Night terrors are also more common in children and subside with age. They are characterized by the sudden onset of out-of-sleep panic and fearful behaviors such as screaming, associated with increased autonomic activation with diaphoresis, tachycardia, tachypnea, flushing, and mydriasis.37 They are distinct from somnambulism due to the presence of profound fear and automonic activation. Patients do not remember the events, and typically no treatment is necessary.
Restless Legs Syndrome
Epidemiology
Restless legs syndrome (RLS) occurs in 1–10% of the population.45,46 However, only 2.5% have symptoms severe enough to necessitate clinical attention and treatment.47 The prevalence increases with age. RLS may also be seen in children, whose symptoms are often confused with ‘growing pains’ or attention deficit hyperactivity disorder (ADHD).46 While more commonly found in Caucasians, RLS occurs in all ethnic groups.46 There is no gender difference, and it is thought be familial, with patterns suggestive of autosomal dominant inheritance.14,46,48
Diagnostic Criteria
Patients with RLS describe unpleasant leg sensations (crawling, tingling, cramping, pain) during recumbency prior to falling asleep.45 Symptoms improve with volitional movements such as walking.45 Diagnosis of RLS is based on the history of unpleasant leg sensations with an urge to move the legs, symptoms worse at night or with prolonged inactivity, symptoms relieved by voluntary limb movement, and difficulty initiating sleep. Many patients with RLS also suffer from periodic limb movement disorder (PLMD) during sleep, which can be a supportive feature in making the diagnosis of RLS. PLMD is characterized by bilateral limb movements in sleep during the first half of the night.45 PLMD must be diagnosed by PSG, with surface EMG monitoring showing repetitive limb movements during non-REM stage 1 and 2 sleep.14,49 The limb movements are often associated with arousals, resulting in chronic sleep disturbance and insomnia. In fact, RLS patients can also exhibit periodic limb movements when awake and may be unable to keep their legs immobile.50Pathophysiology
RLS can be idiopathic or secondary to an underlying medical condition such as pregnancy, iron or folate deficiency, peripheral neuropathy, radiculopathy, or rheumatoid arthritis.45 The pathophysiology of RLS is uncertain, but may be associated with impaired dopaminergic transmission in the nigrostriatal cerebral pathways.51 There is also a strong correlation with iron deficiency, with reduced ferritin levels found in cerebrospinal fluid52 and in serum.53 Even patients with low to normal ferritin <50mcg/l have elevated RLS incidence.54 Magnetic resonance imaging (MRI) and autopsy specimens show reduced iron in the substatia nigra of RLS patients.49
Consequences and Treatment
RLS patients can have significant difficulty falling asleep, multiple awakenings, and reduced total sleep time.54 Often, they also experience diminished quality of life and mood disturbances.55 The primary treatment for RLS is dopaminergic medication, with second-line therapies including gabepentin, benzodiazepines, or opioids.49,54 Pramipexole and ropinerole are commonly used dopaminergic agents in RLS and, as of January 2008, are the only US Food and Drug Administration (FDA)- approved treatment options for RLS.56
Levodopa is also efficacious, but its use is limited by a phenomenon called augmentation, in which RLS symptoms become more severe and occur earlier in the day in a subset of patients, particularly among those with iron deficiency.57 Iron supplementation can be used as an adjunct therapy, but is not considered to be as effective when given as monotherapy (see Table 2).49
* Only US Food and Drug Administration (FDA)-approved drugs for restless legs syndrome as of January 2008. Modified after Avidan and Zee, 2006
Conclusion
In conclusion, sleep disorders have the potential to affect myriad aspects of the lives of patients, with physical, social, and psychological consequences. Therefore, the accurate diagnosis and treatment of sleep disorders should be prioritized as a part of routine medical care.