Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a rare, autoimmune neurological disorder in which peripheral nerve demyelination typically results in weakness, impaired limb sensation, fatigue and pain.1–4 CIDP may adversely affect activities of daily living, with a substantial impact on functional ability and psychological well-being.2–6 Primary treatment goals are reducing symptoms, improving functional status and maintaining long-term remission.7 The most recent joint European Academy of Neurology/Peripheral Nerve Society guidelines recommend corticosteroids or intravenous immunoglobulin (IVIG) as first-line or maintenance treatment options for patients with CIDP and disabling symptoms.8 Subcutaneous immunoglobulin (SCIG) is also recommended as an alternative maintenance therapy in IVIG-responsive patients with active CIDP.8 Although corticosteroids offer several benefits, long-term exposure may cause potentially harmful side effects,9,10 meaning many clinicians and patients choose immunoglobulins as alternative first-line treatments.11
Treatment individualization is an important consideration in CIDP. Patient preferences may vary according to factors such as dosing regimen, mode and logistics of administration, and number of infusion sites, which should be considered to optimize patient satisfaction, convenience and effectiveness.11,12 The different modes of administration of IVIG and SCIG impact on dosing strategies, infusion parameters and treatment optimization.12 IVIG typically permits larger infusion volumes and less frequent dosing but requires venous access and carries an increased risk of systemic adverse events (AEs), mostly related to peak serum immunoglobulin (Ig) G levels.13,14 IVIG is usually administered in a hospital or infusion centre.15 In contrast, SCIG can be administered at home by patients or caregivers without requiring venous access.15 However, with conventional SCIG (SCIG without hyaluronidase), infusion volumes are limited to a maximum of ~30 mL per site, necessitating more frequent dosing and potentially multiple infusion sites and needlesticks per infusion.14
In contrast to conventional SCIG (i.e. without hyaluronidase), HyQvia (Takeda Pharmaceuticals USA, Inc. Lexington, MA, USA), a hyaluronidase-facilitated subcutaneous immunoglobulin (fSCIG), comprises a dual-vial unit of immunoglobulin G (IgG) 10% (GAMMAGARD LIQUID, Takeda Pharmaceuticals USA, Inc. Lexington, MA, USA; Kiovig, Takeda Manufacturing Austria AG, Vienna, Austria) and recombinant human hyaluronidase PH20 (rHuPH20).16,17 The two components are infused sequentially, first with rHuPH20, followed by the subcutaneous infusion of IgG 10% within 10 min.16,17 The recommended rHuPH20 dose is 80 U/g IgG, corresponding to 0.5 mL of rHuPH20 solution per 10 mL of IgG 10% solution.16 The full rHuPH20 dose is infused at 1–2 mL/min (60–120 mL/h) at each site or as tolerated.16
The subcutaneous space is formed by a collagen and elastin network filled with a gel-like substance called hyaluronan, which is partly responsible for resistance to fluid flow through this tissue.18,19 rHuPH20 acts locally to depolymerize hyaluronan, transiently increasing tissue permeability to IgG and allowing larger volumes to be administered and absorbed (Figure 1).14,19–23 rHuPH20 has a half-life activity of ≤20 min, and within 24–48 h, hyaluronan is restored, leaving no observable histopathologic changes in the subcutaneous tissue.20,24–26
Figure 1: Mechanism of action of recombinant human hyaluronidase PH20 in the context of fSCIG 10% infusions20–23
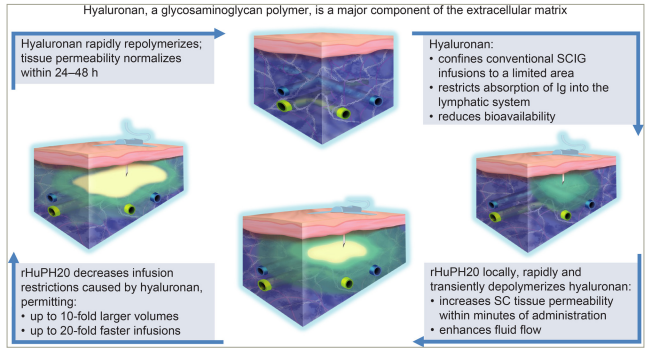
rHuPH20 infusion is shown in green, with IgG 10% infusion shown in yellow and subcutaneous tissue in blue.
IgG = immunoglobulin G; rHuPH20 = recombinant human hyaluronidase PH20; SC = subcutaneous; SCIG = subcutaneous immunoglobulin.
fSCIG 10% combines the benefits of IVIG and conventional SCIG, enabling faster infusion rates and higher doses (meaning less frequent infusions) than conventional SCIG, while allowing home self-administration.18,27 Following findings from ADVANCE-CIDP 1 (A Phase III Study to Evaluate the Efficacy, Safety, and Tolerability of Immune Globulin Infusion 10% [Human] With Recombinant Human Hyaluronidase [HYQVIA/HyQvia] and Immune Globulin Infusion [Human], 10% [GAMMAGARD LIQUID/KIOVIG] for the Treatment of Chronic Inflammatory Demyelinating Polyradiculoneuropathy [CIDP]; ClinicalTrials.gov identifier: NCT02549170) fSCIG 10% has received EU approval as maintenance treatment post-IVIG stabilization in patients with CIDP of all ages and in the USA for adults with CIDP; additionally, fSCIG 10% is approved in the EU as Ig replacement therapy for adults and children with primary immunodeficiency (PID) or secondary immunodeficiency disease and in the USA for PID in adults and children aged ≥2 years.16,17,27
In this article, we examine the potential advantages of fSCIG 10% as a maintenance CIDP therapy versus conventional SCIG and IVIG, as well as providing practical guidance for physicians on use of fSCIG 10% in clinical practice.
fSCIG 10% in CIDP: Comparison with IVIG and conventional SCIG
Efficacy
ADVANCE-CIDP 1 trial
The phase III, double-blind, randomized controlled ADVANCE-CIDP 1 trial evaluated the efficacy and safety of fSCIG 10% in preventing CIDP relapse.27 The study included 132 adults with confirmed CIDP diagnoses who had received stable IVIG for ≥12 weeks before screening. Patients were randomly assigned to either fSCIG 10% or placebo at the same monthly equivalent dose and infusion frequency as their prior IVIG (0.4–2.4 g/kg, maximum 4-week dosing interval) for 6 months or until relapse. The primary outcome was relapse rate, assessed by the proportion of patients experiencing worsening functional disability, defined as a ≥1-point increase from baseline (i.e. pre-subcutaneous treatment) in two consecutive adjusted Inflammatory Neuropathy Cause and Treatment (INCAT) disability assessments obtained ≥7 days apart.27,28 If relapse occurred while receiving fSCIG 10% or placebo during ADVANCE-CIDP 1, patients could enrol in the open-label ADVANCE-CIDP IVIG phase, which assessed IVIG 10% (GAMMAGARD LIQUID/Kiovig) efficacy, safety and tolerability as a rescue treatment in this population.29
ADVANCE-CIDP 1 results showed that fSCIG 10%, when administered at the same dose and interval as prior IVIG, was more effective than placebo at preventing relapse of neuromuscular disability and functional deterioration in CIDP (fSCIG 10% relapse rate: 9.7%, 95% confidence interval [CI] 4.5–19.6%; placebo relapse rate: 31.4%, 95% CI 21.8–43.0%; p=0.0045), with fSCIG 10% reducing absolute risk of relapse by 21.8%. Furthermore, relapse probability was higher with placebo than with fSCIG 10% over the study (Figure 2A; p=0.002), with Kaplan–Meier curves separating early at approximately Week 4.27,30
Figure 2: Kaplan–Meier curves for time to relapse during (A) ADVANCE-CIDP 1 and (B) ADVANCE-CIDP 327,30
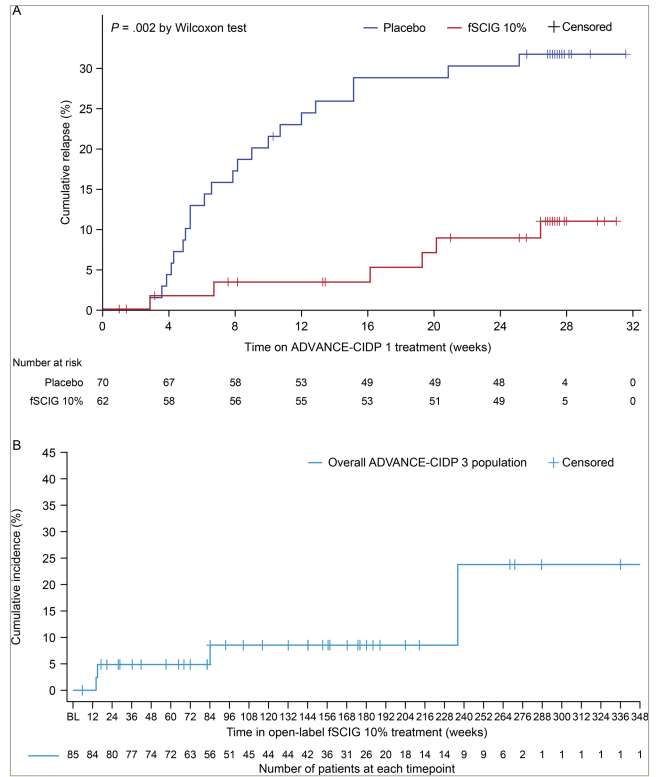
Information in Figure 2 is sourced from the published ADVANCE-CIDP 1 and ADVANCE-CIDP 3 manuscripts, both of which are published as open access under a CC-BY 4.0 license (ADV-1; Bril V, et al. 2023) and CC-BY-NC-ND 4.0 license (ADV-3; Hadden RDM, et al. 2024) and CC-BY-NC-ND 4.0 license (ADV-3).27,30 (A) For ADVANCE-CIDP 1, the curves were estimated using the Kaplan–Meier method for the MITT population, with missing outcomes imputed as no relapse. Time to relapse was calculated as the date of relapse − date of the initial dose of treatment + 1 day. The number of patients who did not relapse was censored with time to censoring calculated as the date of discontinuation or completion − date of initial treatment + 1 day. ADVANCE-CIDP 1 was a double-blind, placebo-controlled phase, in which patients were randomized 1:1 to receive either fSCIG 10% or placebo for a period of 6 months or until relapse. (B) For ADVANCE-CIDP 3, the Kaplan–Meier curve represents the time to relapse during open-label fSCIG 10% treatment, calculated as the date of relapse − date of the initial dose of treatment + 1 day. Patients who did not relapse were censored with time to censoring calculated as the date of discontinuation or completion − date of the initial treatment + 1 day.
BL = baseline; CIDP = chronic inflammatory demyelinating polyradiculoneuropathy; fSCIG = hyaluronidase-facilitated subcutaneous immunoglobulin; MITT = modified intention-to-treat.
ADVANCE-CIDP 3 trial
Patients who completed ADVANCE-CIDP 1 without relapse could enter an open-label extension, ADVANCE-CIDP 3 trial (Long-Term Tolerability and Safety of Immune Globulin Infusion 10% [Human)] With Recombinant Human Hyaluronidase [HYQVIA/HyQvia] for the Treatment of Chronic Inflammatory Demyelinating Polyradiculoneuropathy [CIDP]; ClinicalTrials.gov identifier: NCT02955355), which evaluated the long-term safety and tolerability of fSCIG 10% in patients with CIDP.30 Efficacy was an exploratory outcome. To our knowledge, this was one of the longest follow-up extension studies conducted to date in CIDP, with a median fSCIG 10% exposure of 33 months and up to 77 months for some patients.30 Overall, 86 patients enrolled in ADVANCE-CIDP 3, and 85 received open-label fSCIG 10%, including those who previously received a placebo in ADVANCE-CIDP 1 without experiencing relapse. Patients treated with fSCIG 10% in ADVANCE-CIDP 1 continued at the same dose (mean monthly dose equivalent of 1.1 g/kg, with a maximum 4-weekly administration) until relapse or study end. Patients receiving placebo in ADVANCE-CIDP 1 were switched to fSCIG 10% at the same dose and dosing interval as their previous IVIG. Among patients with non-missing relapse data (n=77), 10 patients (13.0%) experienced relapse, with an overall annualized relapse rate of 4.5%.30 Mean time to relapse was 945.0 days (Figure 2B).30
PATH trial
Data from the PATH trial (Randomized, Multicenter, Double-blind, Placebo-controlled, Parallel-group Phase III Study to Investigate the Efficacy, Safety, and Tolerability of 2 Different Doses of IgPro20 [Subcutaneous Immunoglobulin] for the Treatment of Chronic Inflammatory Demyelinating Polyneuropathy [CIDP] – the PATH Study; ClinicalTrials.gov identifier: NCT01545076) led to conventional SCIG 20% (Hizentra, CSL Behring AG, Bern, Switzerland) receiving approval in the USA and EU for maintenance CIDP treatment (post-IVIG stabilization in the EU).31–34 The PATH trial assessed two different conventional SCIG doses against placebo in 172 patients with CIDP receiving stable IVIG before study entry. Patients were randomized to weekly SCIG 20% (0.2 g/kg, low dose) or 40% (0.4 g/kg, high dose) or placebo for 24 weeks. The primary outcome was the proportion of patients experiencing CIDP relapse (≥1-point increase in adjusted INCAT disability score at any subcutaneous treatment period visit from baseline) or withdrawing from the study for any other reason. Both low- and high-dose SCIG were associated with significantly lower relapse and withdrawal rates than placebo (39%, 33% and 63%, respectively; p=0.001 and 0.007 for high- and low-dose SCIG versus placebo). Patients who completed the study or were rescued from relapse with IVIG could participate in an open-label extension (Multicenter, Open-label Extension Study to Investigate the Long-term Safety and Efficacy of IgPro20 in Maintenance Treatment of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) in Subjects Completing Study IgPro20_3003; ClinicalTrials.gov identifier: NCT02027701).32 This extension provided further evidence that conventional SCIG is an effective maintenance treatment for CIDP, with up to an additional 48 weeks of follow-up (overall relapse rates 10% in high-dose patients and 48% in low-dose patients).32
ICE trial
Between 1993 and 2008, IVIG efficacy in CIDP was established in five randomized, placebo-controlled trials.35–39 Of these, the ICE study (Multicenter, Randomized, Double-blind, Placebo-controlled, Study to Evaluate the Efficacy and Safety of IGIV-Chromatography [IGIV-C], 10% Treatment in Subjects With Chronic Inflammatory Demyelinating Polyneuropathy; ClinicalTrials.gov identifier: NCT00220740) was the largest randomized trial of IVIG in CIDP, leading directly to the approval of GAMUNEX-C® (Grifols Therapeutics LLC, Research Triangle Park, NC, USA).39 This trial assessed the short- and long-term benefits of IVIG versus placebo in 117 patients with CIDP and used baseline loading doses of 2 g/kg administered over 2–4 days, followed by maintenance doses of 1 g/kg over 1–2 days every 3 weeks for up to 24 weeks. The primary outcome was maintained improvement from baseline in the adjusted INCAT disability scale (≥1-point improvement through Week 24). During the first 24-week study period, the patient proportion with improvement in disability was significantly higher with IVIG than placebo (54% versus 21%; p=0.0002), with 60% of patients who had previously received a different IVIG therapy responding by 6 weeks. In a double-blind extension phase (additional 24 weeks), responders from the first study period were eligible to be randomly reassigned to receive either IVIG or placebo. During the extension, patients who continued receiving IVIG had a significantly longer time to relapse than patients receiving placebo (p=0.011). Additionally, the probability of relapse was lower with IVIG than with placebo (13% versus 45%).39 Since the ICE trial, additional IVIG studies have confirmed the benefits of IVIG in CIDP.40–43
Efficacy conclusions
To date, no head-to-head trials have compared relapse rates associated with fSCIG 10% maintenance therapy, IVIG or conventional SCIG. Nonetheless, the relapse rate of 9.7% observed with fSCIG 10% in ADVANCE-CIDP 1 compares favourably with previously reported rates for conventional SCIG and IVIG.27 The smaller absolute effect size with fSCIG 10% (22%) primarily resulted from lower placebo relapse rates in ADVANCE-CIDP 1 (31%) than in the published literature (43–57%).27,31,32,39 These findings could potentially be explained by the lack of mandatory IVIG-dependency testing of patients before randomization in ADVANCE-CIDP 1, which reported a higher probability of CIDP remission than other study populations.27 Caution should always be exercised when comparing results from different studies owing to differing study design (which may not include IVIG-dependency testing), study populations and outcomes. A summary of baseline characteristics and key efficacy outcomes across individual studies discussed above is shown in Table 1.27,30–32,39
Table 1: Summary of key baseline characteristics and primary efficacy outcomes from the active treatment arms of selected clinical trials of fSCIG 10%, conventional SCIG and IVIG therapies in CIDP27,30–32,39
|
| ADVANCE-CIDP 1 fSCIG 10% (n=62)27 | ADVANCE-CIDP 3 fSCIG 10% (n=85)30 | PATH SCIG (IgPro20) 0.2 g/kg (LD) (n=57) 0.4 g/kg (HD) (n=58)31 | PATH extension SCIG (IgPro20) 0.2 g/kg (n=82)32 | ICE IGIV-C 1 g/kg (n=59)39 |
| Study design and treatment | |||||
| Study type | Phase III, randomized, double-blind, placebo-controlled | Phase IIIb, open-label extension (long-term extension of ADVANCE-CIDP 1) | Phase III, randomized, double-blind, placebo-controlled | Phase III, open-label extension (long-term extension of PATH) | Phase III, randomized, double-blind, placebo-controlled, response-conditional crossover |
| Treatment allocation | Patients randomized 1:1 to fSCIG 10% or placebo for 6 months or until relapse or study withdrawal | All patients who completed 6 months in ADVANCE-CIDP 1 received open-label fSCIG 10% (median [range] exposure: 33 [0–77] months) | Patients randomized 1:1:1 to SCIG (IgPro20) 0.2 g/kg (LD), 0.4 g/kg (HD) or placebo for 24 weeks | All patients who completed 24 weeks in the PATH trial received open-label SCIG (IgPro20) 0.2 g/kg for 48 weeks | Patients randomized 1:1 to IGIV-C 1.0 g/kg or placebo for 24 weeks |
| Key baseline characteristics | |||||
| Age, years, mean (SD) | 55.0 (14.3) | 54 (13.1) | LD 58.9 (50.5–66.5)** HD 55.2 (49.2–66.4)** | 57.6 (13.2) | 50 (17) |
| Time since CIDP diagnosis, years, mean (SD) | 4.5 (4.8) | 2.9 (0.9, 16.3)* | LD 2.8 (1.4–5.0)** HD 3.3 (1.3–8.6)** | NR | 2.4 (3.7) |
| Primary endpoint (unless otherwise stated) and outcome | |||||
| Primary endpoint | Proportion of patients experiencing CIDP relapse† | Proportion of patients experiencing CIDP relapse† (exploratory outcome) | Percentage of patients with a CIDP relapse or who were withdrawn for any other reason during 24 weeks of SCIG treatment‡
| Percentage of patients with a CIDP relapse (secondary outcome)§ | Percentage of patients who had maintained an improvement from baseline in adjusted INCAT score ≥1 through Week 24¶ |
| Patients meeting the primary endpoint, n (%) | 6 (9.7) | 10/77 (13.0) | LD 19 (33) (22.5–46.3)*** HD 11 (19) (10.9–30.9)*** | 41 (50.0) | 32 (54) |
*Median (min, max).
**Median (interquartile range).
***95% CI
†The proportion of patients with functional worsening defined as ≥1-point increase relative to the baseline score in two consecutive adjusted INCAT disability scores ≥7 days apart. Relapse status was missing if a patient did not have a baseline INCAT score and at least one post-dose INCAT score or had a missing confirmatory INCAT score in the presence of an abnormal INCAT score within 7 days. In ADVANCE-CIDP 1, the primary endpoint was achieved in 22/70 (31.4%) of patients in the placebo group. In ADVANCE-CIDP 3, the proportion of patients with relapse was 4/39 (10.3%) and 6/38 (15.8%) in the groups that previously received placebo and fSCIG 10%, respectively, in ADVANCE-CIDP 1.
‡Relapse was defined as an increase of ≥1-point in the total adjusted INCAT score at any treatment period visit compared with baseline. The data shown above are from a sensitivity analysis (CIDP relapse analysis) in which all patients who did not experience a relapse were considered non-relapsers (placebo group: 56% [95% CI: 43.3–68.2]). Note, the primary endpoint of PATH was defined as the percentage of patients who experienced a CIDP relapse during SCIG treatment or who were withdrawn from the study during SCIG treatment for any reason; the proportion of patients (95% CI) meeting the primary endpoint: LD, 39% (27.1–51.6); HD, 33% (22.1–45.6) and placebo, 63% (50.2–74.5).
§Relapse was defined as an increase ≥1-point the total adjusted INCAT score compared with baseline. The data shown above relate to all patients in the extension study, regardless of treatment at the end of the PATH study (relapse rate was not reported for patients previously treated with placebo in the double-blind treatment phase of PATH). Note, in the original protocol, a 0.4 g/kg dose of SCIG was used as the initial weekly dose in the extension. Following a protocol amendment, a 0.2 g/kg dose was used as the initial dose (patients who relapsed on the lower dose could opt to switch to the higher dose).
¶Improvement of ≥1-point in adjusted INCAT score at Week 6 versus baseline and maintained at Week 24. The primary endpoint was achieved in 12/58 (21%) of patients in the placebo group.
CI = confidence interval; CIDP = chronic inflammatory demyelinating polyradiculoneuropathy; fSCIG = hyaluronidase-facilitated subcutaneous immunoglobulin; HD = high dose; Ig = immunoglobulin; IGIV-C = immunoglobulin intravenous, 10% caprylate-chromatography purified; INCAT = inflammatory neuropathy cause and treatment; IVIG = intravenous immunoglobulin; LD = low dose; NR = not reported; SCIG = subcutaneous immunoglobulin; SD = standard deviation.
Safety
Systemic adverse events
Systemic AEs associated with subcutaneous therapies are similar to those associated with IVIG but occur less frequently.13 In ADVANCE-CIDP 1, the rate of systemic AEs considered related to fSCIG 10% was 0.11 events per infusion, 1.04 per patient and 2.50 per patient-year.27 Causally related AEs of headache, pyrexia and hypertension were experienced by 6.5%, 4.8% and 3.2% of patients receiving fSCIG 10%, respectively. In ADVANCE-CIDP 3, the rate of systemic AEs considered related to fSCIG 10% was 0.08 events per infusion, 3.38 per patient and 1.30 per patient-year.30 In the PRIMA (A Single-arm Study to Demonstrate the Efficacy and Safety of Privigen in the Treatment of Subjects With Chronic Inflammatory Demyelinating Polyneuropathy [CIDP]; ClinicalTrials.gov identifier: NCT01184846) and ProCID (Prospective, Double-blind, Randomized, Multicenter Phase III Study Evaluating Efficacy and Safety of Three Different Dosages of NewGam in Patients With Chronic Inflammatory Demyelinating Poly[Radiculo]Neuropathy; ClinicalTrials.gov identifier: NCT02638207) trials, which both investigated the effects of IVIG 10% in patients with definite or probable CIDP over 10 and 24 weeks, respectively, headache was reported as the most common causally related AE (28.6% and 14.1%, respectively).42,43 For the PRIMA trial, headache was followed by hypertension and asthenia (both 14.3%), compared with allergic dermatitis (9.2%) and pyrexia (7.7%) in the ProCID trial.42,43
In ADVANCE-CIDP 1, for all AEs, the proportions of patients receiving fSCIG 10% experiencing headache, pyrexia and hypertension were 12.9%, 11.3% and 6.5%, respectively. In the PATH study, headache was experienced by 7.0% and 6.9% of patients receiving low- and high-dose conventional SCIG, respectively.31 In contrast to subcutaneous therapies, in the ICE trial, patients receiving IVIG experienced more frequent systemic AEs, including headache (31.9%), pyrexia (13.3%) and hypertension (8.8%).39
Local adverse events
Local infusion-site reactions, such as redness, itching and swelling, are relatively common and occur more frequently with subcutaneous therapies than with IVIG.11,13 In ADVANCE-CIDP 1, local infusion-site reactions – pain, erythema, pruritis and oedema – occurred in greater proportions of patients receiving fSCIG 10% versus patients on low- and high-dose SCIG in the PATH trial.27,31 For example, injection-site erythema occured in 21.0% of patients receiving fSCIG 10% in ADVANCE-CIDP 1, versus 8.8% and 17.2% of patients on low- and high-dose SCIG in the PATH trial.27,31 The apparently higher rate of local infusion-site reactions with fSCIG 10% than conventional SCIG is presumably because of the higher doses administered, higher infusion rate and faster absorption achieved with hyaluronidase-facilitated administration. However, both studies reported that local-site reactions were mostly mild to moderate in severity and declined over time.27,31 Some specific local AEs are associated with fSCIG 10% owing to high-dose infusion. In ADVANCE-CIDP 1, a patient receiving high-dose fSCIG 10% experienced diffuse abdominal swelling; however, 24 h post-treatment, this had greatly improved without sequelae. Some transient diffuse abdominal swelling is common in most patients after high-dose fSCIG 10% administration at abdominal infusion sites and may be particularly noticeable in patients with slimmer builds. Infusion-site swelling may be less frequently observed in patients with PID owing to the lower fSCIG 10% doses used in this population. To ease potential patient anxiety, setting clear expectations before subcutaneous treatment may be a useful strategy in CIDP.44
A summary of key safety outcomes across individual studies discussed in this article is shown in Table 2.27,30–32,39
Table 2: Summary of key safety outcomes from the active treatment arms of selected clinical trials of fSCIG 10%, conventional SCIG and IVIG therapies in CIDP 27,30–32,39
|
| ADVANCE-CIDP 1 fSCIG 10% (n=62)27 | ADVANCE-CIDP 3 fSCIG 10% (n=85)30 | PATH SCIG (IgPro20) 0.2 g/kg (LD) (n=57) 0.4 g/kg (HD) (n=58)31 | PATH extension SCIG (IgPro20) 0.2 g/kg (n=82)32* | ICE IGIV-C 1 g/kg (n=113)39† |
| Any AE | 49 (79.0) | 76 (89.4) | LD 33 (57.9) HD 30 (51.7) | 62 (75.6) | 85 (75) |
| Any serious AE | 2 (3.2) | 20 (23.5)‡ | LD 3 (5.3)§ HD 2 (3.4) | 7 (8.5)¶ | 6 (5) |
| Systemic AEs | |||||
| Gastrointestinal disorders | 12 (19.4) | NR | NR | 6 (7.3) | NR |
| Nausea | 7 (11.3) | 11 (12.9) | NR | 2 (2.4) | 7 (6) |
| Diarrhoea | 0 | 12 (14.1) | NR | 2 (2.4) | NR |
| Vomiting | 1 (1.6) | 8 (9.4) | NR | 2 (2.4) | NR |
| General disorders | 19 (30.6) | NR | LD 16 (28.1) HD 18 (31.0) | 22 (26.8) | NR |
| Fatigue | 6 (9.7) | 2 (14.1) | LD 5 (8.8) | 4 (4.9) | NR |
| Pyrexia | 7 (11.3) | 17 (20.0) | HD 0 NR | NR | 15 (13) |
| Nervous system disorders | 19 (30.6) | NR | LD 6 (10.5) HD 6 (10.3) | 10 (12.2) | NR |
| Headache | 8 (12.9) | 23 (27.1) | LD 4 (7.0) HD 4 (6.9) | 4 (4.9) | 36 (32) |
| Dizziness | 4 (6.5) | NR | NR | 2 (2.4) | 7 (6) |
| Local AEs | |||||
| Injection-site pain | 10 (16.1) | 5 (5.9) | LD 3 (5.3) HD 2 (3.4) | 3 (3.7) | NR |
| Injection-site erythema | 13 (21.0) | 13 (15.3) | LD 5 (8.8) HD 10 (17.2) | 7 (8.5) | NR |
| Injection-site pruritus | 8 (12.9) | 5 (5.9) | LD 0 HD 2 (3.4) | NR | NR |
| Injection-site oedema | 2 (3.2) | NR | LD 1 (1.8) HD 0 | 9 (11.0)†† | NR |
Data shown are the number of patients with the specified AE (%). It should be noted that direct comparison of the percentage of patients with AEs across studies is complicated by differences in the duration of follow-up and data reporting between study publications. In general, studies with a longer duration of follow-up (e.g. ADVANCE-CIDP 3) generally show higher rates of AEs.
*Data shown are across all treatment groups. Note that in the original protocol, a 0.4 g/kg dose of SCIG was used as the initial weekly dose in the extension. Following a protocol amendment, a 0.2 g/kg dose was used as the initial dose (patients who relapsed on the lower dose could opt to switch to the higher dose).
**Occurred at a rate of 24 events per 100 fSCIG 10% infusions.
†Safety data were pooled from each period of the ICE study, comprising 113 patients exposed to IGIV-C and 95 exposed to placebo. The efficacy data reported in Table 1,relate to the first period of the study (i.e. the 24-week double-blind treatment phase).
††Reported as injection-site swelling.
‡Only three serious AEs were considered to be treatment related.
§Only one serious AE (in the LD group) was considered to be treatment related.
¶None of the serious AEs were considered to be treatment related.
AE = adverse event; CIDP = chronic inflammatory demyelinating polyradiculoneuropathy; fSCIG = hyaluronidase-facilitated subcutaneous immunoglobulin; HD = high dose; Ig = immunoglobulin; IGIV-C = immunoglobulin intravenous, 10% caprylate-chromatography purified; LD = low dose; NR = not reported; SCIG = subcutaneous immunoglobulin.
Immunogenicity
ADVANCE-CIDP 1 examined the immunogenic potential of rHuPH20.27 Overall, eight patients (6.1%) developed positive binding anti-rHuPH20 antibodies (titre ≥1:160), which were not associated with an increased incidence of AE, including local or systemic reactions.27 No patients developed neutralizing antibodies.27 In ADVANCE-CIDP 3, overall, 14 patients (16.7%) with available data had at least one positive anti-rHuPH20 antibody titre (≥1:160) during treatment, consistent with those previously reported for other subcutaneous treatments co-administered with rHuPH20 (0.9–44.7%), and in healthy IgG donors without rHuPH20 exposure (1.6–12.1%).30,45–47 Among these 14 patients, 2 (2.4%) had neutralizing antibodies (titre ≤1:100).30 Again, antibody positivity was not associated with increased AE incidence, with AE rates per patient-year of 7.8 and 6.0 in the presence and absence of treatment-emergent anti-rHuPH20 antibodies, respectively.30 Additionally, patients who tested positive for binding anti-rHuPH20 antibodies had similar relapse rates to patients who tested negative (2 [16.7%] versus 8 [12.3%] patients, respectively).30 Although there is a possibility of these antibodies developing following immune response to rHuPH20, patients receiving IgG infusions may test positive owing to the passive transfer of antibodies that are present in the IgG component of fSCIG 10%.30,46 While IgG therapies prepared from pooled donor plasma may contain rHuPH20-reactive antibodies, routine measurement of anti-drug antibodies against hyaluronidase is not warranted in clinical practice owing to the lack of clinical significance.30 These results from ADVANCE-CIDP 3 are consistent with fSCIG 10% studies in PID, as well as with an extensive review of different therapeutics using rHuPH20 for several diseases.19,46
Infusion characteristics
fSCIG 10% represents an important alternative to IVIG and conventional SCIG in CIDP. fSCIG 10% allows subcutaneous infusion of large doses at high infusion rates (maximum volume 1,200 mL [120 g] per day in two or three sites), and thereby permitted less frequent infusions and fewer infusion sites without requiring venous access in ADVANCE-CIDP 1.27 For patients receiving fSCIG 10%, the median (range) monthly dose equivalent was 82.6 (27–217) g. Patients receiving fSCIG 10% (88.7%) had a 4-week dosing interval, and 3.2% and 8.1% of patients were dosed every 2 or 3 weeks, respectively.27 The pre-specified maximum total doses and volumes that could be administered in a single day were up to 120 g/1,200 mL for patients weighing ≥40 kg and 60 g/600 mL for those <40 kg, respectively.27 Some patients (17/132 [12.9%]) had fSCIG 10% administered over 2 days owing to tolerance issues or the dose for infusion; in clinical practice, all patients should be able to receive single-day treatment by infusing smaller doses more frequently. The average time to deliver fSCIG 10% was about 2 h, approximately half of the 3–5 h average infusion time for the nearly equivalent IVIG dose.11,27 In ADVANCE-CIDP 3, the median (range) monthly dose equivalent was 69.6 (30.4–209.4) g, and the infusion volume was 620.0 (50.0–1200.0) mL.30 Most patients had a 4-week (88.2%) or 3-week (11.8%) dosing interval, and most used two infusion sites (92.3%; 2.7% and 5.0% used one and three sites, respectively).30 Only 14.1% of patients (12/85) required 2 days to complete fSCIG 10% infusion.30 Table 3 summarizes treatment attributes and infusion characteristics of fSCIG 10%, IVIG and conventional SCIG.11–13,16,17,33,48
Table 3: Comparison of treatment attributes and infusion characteristics of fSCIG 10%, IVIG and conventional SCIG therapies in CIDP11–13,16,17,33,48
| Infusion attribute or characteristic | Hyaluronidase-fSCIG 10% | IVIG 10% | Conventional SCIG 20% |
| Infusion setting | May be self-infused at home or other convenient locations | Hospital or infusion clinic or at home with infusion nurse support | May be self-infused at home or other convenient locations |
| Maximum infusion volume per site, mL (g)* | 600 (60 g, if one to two infusion sites) 400 (40 g, if three infusion sites) | NA | 25–50 (6–10 g) |
| Number of infusion sites† | 1–3 | 1 | 1–8 |
| Dosing intervals, weeks‡ | 2–4 | 2–8 | 1 |
| Infusion rate | For patients weighing ≥40 kg: maximum infusion rate of 240 mL/h/site for initial one or two infusions, increasing up to 300 mL/h/site for subsequent infusions, as tolerated For patients weighing <40 kg: maximum infusion rate of 80 mL/h/site for initial one or two infusions, increasing up to 160 mL/h/site for subsequent infusions, as tolerated | 0.3 mL/kg/h for initial infusions, increasing up to ≤4.8 mL/kg/h, as tolerated | ≤20 mL/h/site for initial infusions, increasing up to ≤50 mL/h/site, as tolerated |
| Approximate infusion duration, h | 2 | 3–5 | 1–1.5 |
| IgG level profile | Dependent on dosing regimens; for regimens with lower doses and shorter dosing intervals, IgG level profile is near steady state; for higher doses and longer intervals, the profile is cyclical but with shallower troughs and peaks than IVIG | Cyclical, troughs and peaks | Near steady state |
*fSCIG 10% can be administered at one, two or three infusion sites with a maximum infusion volume of 600 mL/site (or as tolerated; maximum daily infusion volume 1,200 mL for patients weighing ≥40 kg); if using three sites, the maximum volume of fSCIG 10% is 400 mL/site (maximum daily infusion volume 1,200 mL for patients weighing ≥40 kg). Maximum infusion volume is dependent on patient tolerability.
†The number of infusion sites with SCIG 20% will depend on doses administered.
‡Dosing of SCIG 20% may be spread optionally across 1–3 days/week.
fSCIG = hyaluronidase-facilitated subcutaneous immunoglobulin; IgG = immunoglobulin G; IVIG = intravenous immunoglobulin; NA = not applicable; SCIG = subcutaneous immunoglobulin.
Patient-reported outcomes in clinical trials
In ADVANCE-CIDP 1, patients receiving fSCIG 10% maintained Short Form-36 Health Survey (SF-36) and EuroQol-5 Dimension (EQ-5D-3L) scores, while patients receiving placebo showed slight worsening in these scores.27 Additionally, global satisfaction scores on the 9-item Treatment Satisfaction Questionnaire for Medication (TSQM) were assessed, with higher scores reflecting increased satisfaction.27 Global satisfaction scores were higher in patients receiving fSCIG 10% than placebo (65.3 versus 55.7, respectively).27 Results from a treatment preference questionnaire indicated that most patients receiving fSCIG 10% (66.7%) and placebo (70.6%) preferred the study treatment over their previous IVIG, and most patients in both groups stated that they would choose to continue receiving their allocated treatment after study end (fSCIG 10%: 83.3%, placebo: 92.2%), rather than revert to IVIG.27 In ADVANCE-CIDP 3, patients receiving fSCIG 10% generally maintained SF-36 and EQ-5D-3L scores over the study.30 At treatment end, 9-item TSQM global satisfaction scores were also maintained, and 84.1% of patients indicated a favourable overall preference for fSCIG 10% over their previous IVIG treatment.30
In the PATH trial, health-related quality of life measures showed better patient outcomes with low- and high-dose SCIG over placebo, using the EQ-5D and 14-item TSQM measures.31 Overall, 53% of patients receiving SCIG preferred their current treatment over prior IVIG versus 39% of patients receiving placebo.31
Costs of different immunoglobulin therapies
Ig dose and formulations are the primary cost drivers of CIDP treatment.11,13 Costs of immunoglobulins vary in widely among different countries, can change rapidly over time and are further complicated by indirect costs associated with site of care, healthcare professional (HCP) resources and requirement for hospitalizations,13 which also differ by country. The different bioavailability across administration routes may affect the dose required, and therefore impacting the associated cost. The cost per gram of SCIG is typically higher than that of IVIG; however, the overall cost may be offset by infusion cost savings, reduced productivity loss and reduced costs associated with missed infusion appointments.13,49,50 It remains unclear which administration route will prove the most cost-effective overall, and further research is warranted to compare the costs of immunoglobulins in CIDP.13
To date, the USA has the highest Ig usage per capita, followed by Canada, Australia and some European countries.51 The use of conventional SCIG is growing faster than IVIG, and in 2019 in the USA, SCIG represented 15% of total Ig use and 61% of Ig therapies for patients with PID.51
Pharmacokinetics and bioavailability
fSCIG 10% uses rHuPH20 to depolymerize subcutaneous hyaluronan, transiently increasing tissue permeability and allowing for the administration of larger doses and less frequent infusions than with conventional SCIG.27 Conventional SCIG is administered more frequently (typically weekly) and in smaller doses, resulting in steadier IgG concentrations between infusions and lower peak serum IgG concentrations than with IVIG.12 IVIG is directly administered into the bloodstream, resulting in rapid increases in IgG levels, which may lead to faster improvements in disability and may also cause adverse effects, such as headache.13 Towards the end of an IVIG dosing cycle, lower IgG trough levels can result in cyclic ‘wearing-off’, which worsens disease symptoms in some patients, especially those with longer dosing intervals.12,13 These ‘wear-off’ effects may also occur in some patients receiving fSCIG 10%, although further study is required; however, they are rare with conventional SCIG.
The bioavailability of different Ig therapies varies according to administration route, which may affect the dose required.11 IVIG has a bioavailability of 100% and conventional SCIG of approximately 63–69%, whereas fSCIG 10% offers a bioavailability of >90% and IgG trough levels comparable with those with IVIG.21,27,52,53 To maintain comparable IgG trough concentrations, higher doses of conventional SCIG are typically required than fSCIG 10% owing to its incomplete absorption; this may offset cost differences per gram, making fSCIG 10% more cost-effective.
SCIG 10% in CIDP: Guidance for clinical practice
This section focuses on practical guidance for clinicians regarding fSCIG 10% initiation and transitioning from other Ig therapies. It also discusses specific considerations for fSCIG 10% administration, associated patient benefits and pertinent challenges and learnings from ADVANCE-CIDP 1 and 3.
Initiating fSCIG 10%
In the USA, patients should have received stable doses of IVIG (or conventional SCIG) before initiating fSCIG 10%.16 Maintenance doses and dosing frequency of fSCIG 10% should usually be the same as previous IVIG, except for patients with IVIG dosing intervals of >4 weeks, for whom intervals can be converted into 2, 3 or 4 weeks while maintaining the same monthly equivalent IgG dose.16 A ramp-up schedule is recommended for initiating fSCIG 10% (except for low-dose treatment, e.g. ≤0.4 g/kg), starting with more frequent, smaller doses (at the same weekly equivalent dose) to ensure patient tolerability (this can take up to 2–9 weeks depending on a dosing interval, tolerability and clinical discretion).16 Dose and dosing frequency can be adjusted later based on the clinical response.16
In the EU, the typical dosing interval for fSCIG 10% is 3–4 weeks, and the recommended dose is 0.3–2.4 g/kg monthly, administered over 1 or 2 days.17 Patient clinical response should be the primary consideration in dose adjustment. In cases of clinical deterioration, the dose may be increased, potentially up to the recommended maximum of 2.4 g/kg monthly.17 Dose ramp-up schedules are also recommended to ensure patient tolerability until the full target dose is reached, except for lower doses of ≤0.4 g/kg.17 When transitioning to fSCIG 10%, patients must have been on stable IVIG doses, and the US label considerations for patients transitioning to fSCIG 10% also apply.17
Practical patient-related considerations for fSCIG 10% administration
There are several considerations when choosing fSCIG 10%. Some may be informed by patient preference, such as dosing regimens, number of infusion sites and use of a pump. Other factors, such as the use of subcutaneous needles, may be new to patients previously treated with IVIG or challenging for individuals uncomfortable with needles and may require additional counselling and support.
For many patients with CIDP, fSCIG 10% can be self-administered at home without HCP supervision after adequate training and can provide patients with a degree of autonomy, convenience and flexibility, which may lead to improved treatment adherence and better outcomes.54 Patients require self-administration training from a nurse, typically for two to five sessions, before becoming competent independently. fSCIG 10% typically offers a lower treatment burden than conventional SCIG because it enables less frequent infusions and requires fewer infusion sites for equivalent monthly doses.27 This makes it particularly attractive for patients receiving higher Ig doses. Although home administration offers significant convenience, patients may still benefit from frequent follow-ups, either via in-person clinic visits or by phone, to assess treatment response, identify any treatment barriers, identify and discuss any treatment-related side effects and monitor adherence.44 For successful long-term adherence, patient support programmes would benefit patients transitioning to fSCIG 10%.13
The opportunity for home self-administration of fSCIG 10% eases the burden of travel, loss of work and associated expenses. It may be more convenient for patients with scheduling and/or logistical issues when attending hospital appointments, particularly those living far from their infusion clinic or with demanding work or home life commitments.13 However, in a home setting, patients and caregivers have greater responsibility for managing infusions. Some patients may struggle to self-administer at home owing to poor manual dexterity, particularly those without reliable support networks.13 Others may not wish to medicalize their home environment or may be uncomfortable with needles, preferring a clinical setting instead.12 Therefore, it is vital for patients to have ongoing support and good communication with HCPs.
HCPs have an important role in helping patients understand the potential benefits of transitioning to fSCIG 10% and in developing realistic patient expectations. HCPs offer advice regarding the intensity and frequency of local-site reactions and how to manage them, and provide advice on infusion techniques and optimization. fSCIG 10% infusions are typically infused into the abdomen but may also be infused into the thigh. Dividing infusions across two or three simultaneous sites (using a bifurcated or trifurcated tube from a single infusion pump) may improve tolerability and speed by reducing infusion volume per site. Shortening fSCIG 10% dosing intervals, when needed, may allow patients to receive their required dose in a single day rather than two, resulting in fewer treatment days per month.
In certain populations, fSCIG 10% may offer a particularly useful treatment option. Establishing peripheral venous access may be problematic in older patients, and central venous line insertion may pose additional risks with respect to infection and device maintenance, meaning subcutaneous treatments are preferred.13,55–58 Additionally, subcutaneous treatments offer an alternative for patients with a history of systemic general reactions to IVIG, such as patients with anti-immunoglobulin A (IgA) antibodies and unmeasurable serum IgA levels. Based on the authors’ experience, patients experiencing relapse after surgery or viral illness and a previous period of clinical stability were able to remain on their stable fSCIG 10% dose while receiving an additional single IVIG cycle for symptom control.
Challenges, limitations and learnings from ADVANCE-CIDP 1 and 3
ADVANCE-CIDP 1 experienced challenges and limitations common to recruitment in CIDP trials, such as low disease prevalence and patient unwillingness to discontinue therapy. Patients received stable IVIG for at least 12 weeks before the study but did not undergo pre-randomization IVIG-dependency testing. Therefore, the trial population may have had a higher probability of CIDP remission than other randomized controlled trials that used IVIG-dependency testing, which could explain the lower relapse rates observed with both fSCIG 10% and placebo versus other studies.27 Including IVIG-dependency testing is recommended for future CIDP clinical trials. ADVANCE-CIDP 3 had an inherent selection bias for patients willing to continue fSCIG 10% and who did not experience relapse; therefore, patients experiencing AEs may have dropped out, and it could be expected that AE rates would reduce over time.
All patients receiving long-term home treatment should still have regular follow-up visits with the treating physician. Attending physicians should consider trialling dose reduction after a period of clinical stability to assess whether patients have entered remission as for IVIG, with dose adjustments based on a combination of neurological examination, patient-reported symptoms and clinical response.12 Administration frequency and dose/cycle should be individualized for each patient and regularly reassessed to adapt treatment to meet patients’ needs.
Conclusions
fSCIG 10% has demonstrated efficacy as a maintenance treatment with favourable long-term safety and tolerability in patients with stable CIDP switching from IVIG. fSCIG 10% combines the benefits of IVIG and conventional SCIG: the convenience of potential home self-administration, at a similar dose, volume and frequency to IVIG, and a lower risk of systemic AEs. Most patients preferred fSCIG 10% over their prior IVIG, and compared with conventional SCIG, it has greater bioavailability. fSCIG 10% is likely to be more convenient than conventional SCIG for patients with CIDP, especially those requiring higher Ig doses.
Plain language summary
In chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), the immune system attacks the nerves in a patient’s legs and arms. This may be treated by proteins that help control the immune system, called immunoglobulins (Ig). Ig treatments can be given either as intravenous immunoglobulin (IVIG), subcutaneous immunoglobulin (SCIG), or hyaluronidase-facilitated subcutaneous immunoglobulin (fSCIG) 10%. All of these treatments appear to treat CIDP equally well. SCIG may be administered at a patient’s home, and generally causes fewer adverse events than IVIG. fSCIG 10% can be administered less often (typically every 4 weeks) and with fewer needles than SCIG. Results from the ADVANCE-CIDP 1 and ADVANCE-CIDP 3 studies showed that patients with CIDP preferred fSCIG 10% over IVIG. When patients switch treatments to start receiving fSCIG 10%, doctors must consider dosing information and carefully monitor patients after the change in therapy. Treatment should be adapted to best meet the patients’ needs.













