Chronic inflammatory demyelinating polyneuropathy (CIDP) is a peripheral neuropathy that can affect motor and/or sensory nerves, and lead to both loss of strength and altered sensation.1 Common first-line treatments for CIDP are immunoglobulins (Igs), corticosteroids and plasma exchange.2 Intravenous immunoglobulins (IVIgs) have been shown to be efficacious in maintaining or improving strength and disability in a number of CIDP studies.3,4 Limitations of IVIg include relatively frequent systemic adverse events (AEs), the most common of which is headache.3,4 In addition, although some companies offer home-based IVIg infusions, in most countries the majority of IVIg infusions are given within hospitals or infusion centres.3,5,6 Furthermore, while IVIg is an established treatment for CIDP, it may be associated with fluctuations in functionality throughout the treatment cycle.7
An alternative to IVIg is subcutaneous immunoglobulin (SCIg). SCIg is preferred by many patients because it allows for convenient home-based self-administration, reducing the need for hospital or infusion centre-based treatments. Subcutaneously infused Igs slowly enter the vascular space, with maximum serum immunoglobulin G (IgG) levels reached approximately 2 days after infusion,8 compared with IVIg which enters the vascular space within several hours;9 the slower diffusion of SCIg likely leads to the observed fewer systemic AEs such as headache.10–12 Additional comparisons between IVIg and SCIg are shown in Table 1.13 Of note, due to dedicated manufacturing steps designed to remove or inactivate viruses and other pathogens, both IVIg and SCIg present a very low risk of disease transmission.14–16 Indeed, to date there have been no cases of transmission of viral infection or Creutzfeldt-Jakob disease associated with the use of currently available Ig products. In addition, no cases of progressive multifocal leukoencephalopathy (JC virus reactivation) have been seen with IVIg or SCIg. Here we detail a case study of a patient switching from IVIg to SCIg and review the relevant literature from the neurologist, nurse and patient perspective, including details on the recent large-scale Polyneuropathy And Treatment with Hizentra (PATH) study.10
Patient case study
The patient became ill with acute Guillain-Barré syndrome in 1989. After achieving a partial recovery, he was left with significant disability; although his core strength slowly improved over the next few years. In 2005, he experienced symptoms including weakness in the arms and legs, ‘pins and needles’, neuropathic pain and difficulty walking, and was referred to a neurologist. Raised cerebrospinal fluid protein measurements and overall progression of disability over >2 months, combined with meeting the European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS) criteria for CIDP, resulted in a diagnosis of CIDP. This was further supported by electromyography results consistent with CIDP the following year. The patient was treated with IVIg (2005–2011), SCIg administered via pump (2012–2014) and manual push SCIg (2014–present). A summary of the patient’s experience with all three methods is shown in Box 1.

After IVIg treatment the patient felt considerably stronger, walking ability and manual dexterity improved and pain levels were reduced; although wear-off of treatment effects was experienced between infusions. It was common to experience systemic AEs at the end of the first day of infusions including headache, stomach ache and nausea; however, these did not persist.
Whilst the patient was initially treated with IVIg, when his hospital began offering SCIg, the patient opted for this administration method. With SCIg administered via pump, the patient and his caregiver were trained in the preparation and administration of SCIg. This patient was unable to draw up the product into the syringe; however, once the product was drawn up into the syringe, the rest of the administration process could be undertaken by the patient. The patient did not experience any noticeable AEs with this treatment, with only transient swelling at the site of infusion. With the manual push SCIg method, the patient was able to perform all steps of the infusion without assistance. Stabilisation of symptoms was similar with both SCIg administration methods. Whilst the patient initially experienced some wear-off over time, efficacy was stabilised by increasing the SCIg dose, leading to efficacy similar to peak IVIg. On SCIg, the patient experienced reduced wear-off effects compared with IVIg.
Evidence of subcutaneous immunoglobulin efficacy in neuropathies – a neurologist’s perspective from the literature
Treatment efficacy is a major consideration for neurologists when choosing a suitable treatment for patients with CIDP. Several studies have demonstrated the efficacy of SCIg in neuropathies. Markvardsen et al. recently reviewed 20 publications on SCIg in CIDP or multifocal motor neuropathy (MMN) and concluded that muscle strength, function and ability can be maintained with SCIg treatment in both disorders. SCIg was also associated with fewer systemic AEs compared with IVIg.17 A meta-analysis conducted by Racosta et al. analysed data from eight studies, with a total of 138 patients (50 with MMN and 88 with CIDP).18 No significant difference was observed in muscle strength outcomes between SCIg and IVIg, but an improved AE profile was observed in patients with MMN and CIDP receiving SCIg compared with IVIg.18 Long-term response to SCIg has recently been reported by Cirillo et al., who demonstrated improvement of global strength, sensory deficits and overall disability in patients with CIDP treated with SCIg for 12 or 24 months.19
The PATH study
The recent PATH study (ClinicalTrials.gov identifier: NCT01545076) confirmed the efficacy of SCIg as maintenance therapy in CIDP in a large-scale, placebo-controlled, randomised setting.10 This led to the approval of the 20% SCIg IgPro20 (Hizentra®, CSL Behring, King of Prussia, PA, USA) for maintenance treatment of CIDP by authorities in several countries, including by the US Food and Drugs Administration, Health Canada and the European Medicines Agency.14,15,20
Prior to randomisation to either weekly 0.2 g/kg or 0.4 g/kg SCIg (IgPro20) or placebo for 24 weeks, patients underwent an IgG dependency period and IVIg restabilisation period to confirm their need for, and response to, IgG therapy. The primary endpoint of rate of relapse (≥1-point deterioration in adjusted Inflammatory Neuropathy Cause and Treatment [INCAT] score) or withdrawal was 33% for 0.4 g/kg IgPro20 and 39% for 0.2 g/kg IgPro20, both significantly lower (p=0.001, p=0.007, respectively) than the 63% for placebo. Efficacy measures such as INCAT, grip strength, Medical Research Council sum scores and Inflammatory Rasch-built Overall Disability Score (I-RODS) remained more stable with IgPro20 compared with placebo.10
The most common AEs were local reactions at the infusion site and these were mostly mild in severity and decreased over time. The rate of headache was low in the PATH study (7% of patients treated with SCIg experienced headache compared with 3.5% of placebo-treated patients.
Assessing efficacy with home-based subcutaneous immunoglobulin
When assessing the efficacy of SCIg, ensuring patients adequately monitor their response to treatment with a home-based therapy can be a challenge. Measuring serum IgG levels as a correlate with response does not appear to be beneficial from the PATH study results; however, training in the use of Vigorimeters, or other grip strength devices such as a Jamar handgrip dynamometer, and completion of patient-reported outcome measures such as the I-RODS questionnaire at home can assist in self-monitoring.3,21–23 It is also important to reassure patients that small fluctuations in functionality are common and do not necessarily indicate relapse. Development of new outcome measures that are more suited to home-based, patient-led assessment may be useful for the ongoing use of SCIg in CIDP.
Practicalities of subcutaneous immunoglobulin – a nurse’s perspective
SCIg can be administered via the manual push method or using a pump. Different pumps have advantages and disadvantages including size/portability, infusion rate and volume limits, or added features (e.g., blockage alarms). Two of the most common pumps available are the Crono (Cané, Rivoli, Italy) and the Freedom (RMS Medical Products, Chester, NY, USA) pumps (Figure 1). The battery-powered Crono pump is small and portable (pocket-sized); the infusion speed can be modified, an infusion time is set and it will infuse in that time. The Crono pump requires some dexterity and sensitivity to change the settings, which may be a challenge for some patients. The Freedom pumps are larger and are based on a wind-up mechanism rather than battery power. The speed of infusion is regulated by the lumen (and therefore the pressure) of the rate tubing; infusions can start with a lower lumen/pressure to get patients used to the infusion and then build up if well tolerated. Other pumps are available, such as the SCIg 60 infuser (EMED, El Dorado Hills, CA, USA), and it is important to discuss the range of administration options available to patients to ascertain the best fit for each patient’s lifestyle and personality.
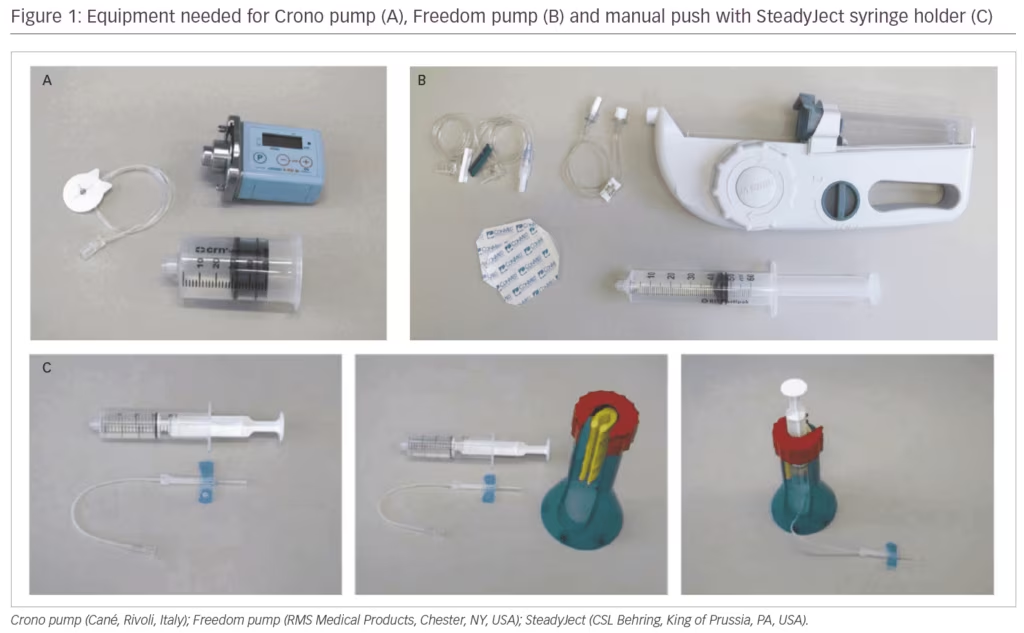
As switching from IVIg to SCIg can mean a change from hospital- or clinic-based administration to home-based self-administration, ensuring that patients are infusing correctly can be a challenge. For patients with dexterity issues, syringe holders can be used to make infusions easier to manage; the availability of pre-filled syringes in certain regions has been shown to be of great benefit to patients with dexterity issues to reduce errors in preparation of infusion equipment.24 One of the major advantages when switching from IVIg to SCIg is the avoidance of IV infusions; SC infusions require minimal training, can be completed by patients in their own homes and ensure that patients with IV access issues can benefit from Ig therapy.25,26 In the PATH study, most patients found the subcutaneous administration technique easy to use and all patients or caregivers learnt to effectively administer IgPro20 or placebo by themselves after four or fewer training sessions.27 Resources such as the International Nurses Group for Immunodeficiency guidelines on Ig administration and Train the Trainer video,28 as well as the Hizentra.com self-administration documents and videos29 can support training in SCIg use. Often a specialty pharmacy provider will train patients on using SCIg in the home over 3–5 sessions and may assess patients via clinic follow-ups or phone assessments to monitor dose, volume, rate, tolerability, compliance and treatment barriers.
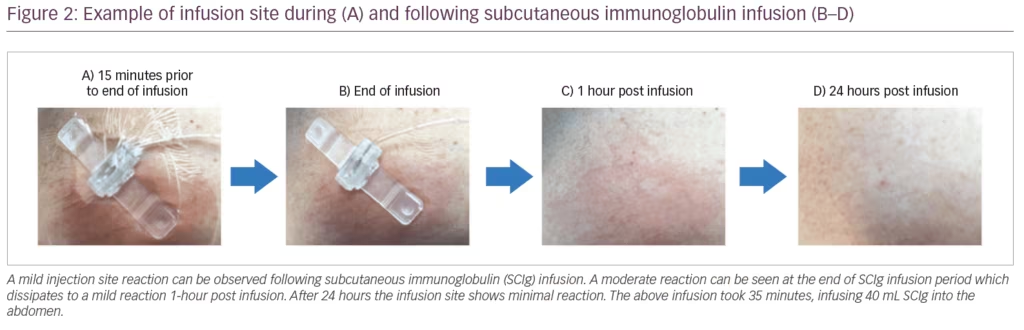
Suitable infusion sites for SCIg are the abdomen, thigh, upper arm, and/or lateral hip, staying ≥2 cm (≥1 inch) away from any scar tissue. It is often recommended to rotate the site of infusion so it is not in the same location as the previous site; however, this is not always necessary and can be based on patient preference. If infusing at multiple sites at once, these sites should be ≥2 inches (5 cm) apart. In the PATH study, the maximum infusion volume per site was 50 mL (≤20 mL/site for the first infusion), and patients used a maximum of eight infusion sites in parallel, most, however, used between two and four needles.10,30 The maximum infusion rate per hour per site in the PATH study was 50 mL (≤20 mL/hour/site for the first infusion), SCIg infusions can therefore last less than an hour. SCIg dosing can be divided and given at various intervals including daily to weekly or every other week with the same benefit as IVIg; however, some patients may require increases in their dosing compared with their previous IVIg dose. When discussing sites with patients, it is important to highlight that a site is usually considered a 2.5 cm2 area, approximately 1 cm around the site of injection, rather than an exact needle position. Transient local reactions can occur following SCIg infusion (Figure 2), these reactions usually subside within 24 hours. If local reactions such as redness, swelling or itching occur during infusion, the infusion can be paused while a heat pack is applied to help with absorption of SCIg and reducing the reaction (redness from the heat application may be present following this and will fade after a short time). Alternatively, changing ancillary supplies such as the tape, transparent dressing, or skin preparation supplies may be suggested. Additionally, further training to confirm technique and optimise needle length, infusion volume and flow rate and infusion site selection may be useful.
Patient Perspectives
From patient-reported outcomes in the literature, the benefits of SCIg versus IVIg appear to include reduced systemic AEs, the option to split infusions over different sessions or multiple sites (flexible dosing) and reduction of wear-off effects (Table 1).13,31 In addition, compared with IVIg (5–6 hours per infusion), SCIg allows for rapid infusion (approximately 1 hour per infusion), which can be tailored to each patient’s tolerance.14,32,33 The preference of IVIg versus SCIg will often depend upon a patient’s individual personality and lifestyle; some patients may prefer to have IV infusions in a healthcare setting and enjoy the social aspect of infusion centres, others may prefer the convenience and autonomy of SCIg. The patient in this case study experienced reduced wear-off effects when switching from IVIg to SCIg, potentially due to more stable serum IgG levels with SCIg. SCIg provides more constant IgG levels compared with IVIg resulting in reduced wear-off effects at the end of treatment cycles.14,34–36 During the PRIMA (Privigen® Impact on Mobility and Autonomy) trial, peaks of 32.3 (SD 8.0) g/L and trough levels of 17.5 (3.1) g/L were seen with IVIg 1 g/kg every 3 weeks,4 whilst in PATH, patients experienced serum IgG trough levels of 15.3 (2.57) g/L at a dose of 0.2 g/kg and of 20.8 (3.23) g/L with 0.4 g/kg.14
It should be highlighted that although the patient in our case study had a positive experience with SCIg, this would not be the ideal setting for patients who prefer fewer infusions or the hospital/clinic setting. The use of implanted ports for IVIg administration can be associated with complications, so SCIg administration may be more appropriate for patients with venous access issues. In a study by Hadden et al. in eight subjects (four with MMN and four with CIDP) who switched from IVIg to SCIg there was a strong preference for SCIg over IVIg, with perceived benefits of SCIg including travel convenience and ease of self-administration.37 In a study by Cocito et al., switching to home-based SCIg improved quality of life index scores; the biggest improvements were seen for ‘treatment not interfering with work’, ‘no anxiety’, ‘autonomy’, ‘convenience’ and ‘painless’ domains after 6 months, suggesting these aspects can impact preference.11 In the PATH study, 53% of patients preferred current SCIg treatment over pre-study IVIg treatment, while approximately 18% preferred IVIg.10 The difference in preference in the PATH study was largely due to patients reporting that SCIg offered them more independence and autonomy, while patients who preferred IVIg did so primarily due to a better perceived efficacy of IVIg.10
Conclusions
SCIg is a suitable alternative to IVIg for the treatment of CIDP in terms of efficacy and tolerability with individual patient preference a key factor in the choice of use of IVIg or SCIg, as shown in the real-world case study presented here and the literature reviewed. In comparison with IVIg, SCIg is associated with fewer systemic AEs; if local reactions are experienced, these tend to be mild and become less frequent over time. Switching from IVIg to SCIg is simple, with different administration options to suit patient needs and limited patient training required from nurses. In spite of weakness, numbness and manual dexterity limitations in patients with neuromuscular diseases, most patients with MMN or CIDP are able to perform subcutaneous injections either themselves or with help from their caregiver, giving patients more independence than with hospital-based IVIg. Despite these advantages, some patients may not tolerate SCIg or show adequate clinical response to SCIg; therefore, close monitoring when switching or starting a patient on SCIg is recommended. Additional clinical research into the use of SCIg in the management of patients with chronic inflammatory neuropathies and other neuromuscular conditions is recommended. ⬛













