Intracranial radiosurgery, no matter the means or methods of administration, is predicated on a core set of principles, including head immobilization and precise delineation of the treatment target. For some five decades after Leksell introduced the concept of stereotactic radiosurgery in 1951,1 rigid head fixation via an invasive device was an integral component towards these ends. However, advances in computer engineering, radiologic technology and radiosurgical techniques provided opportunities to overcome the limitations of the conventional frames. Several excellent reviews have discussed the evolution towards frameless radiosurgery in terms of commercially available systems.2,3 In this article we provide a more comprehensive review of the myriad devices described over the years and the evolutionary pressures that spurred their creation.
Early non-invasive frames
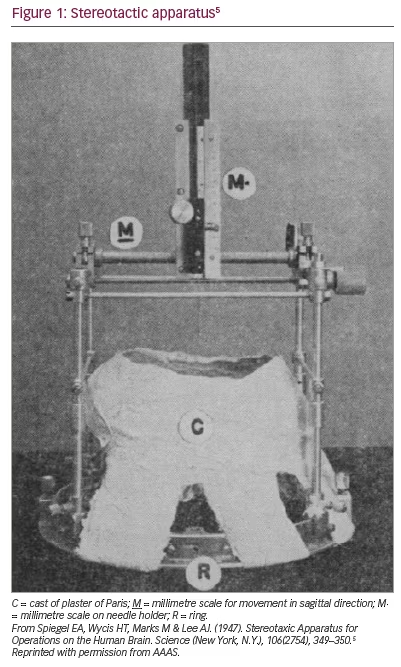
Horsley and Clarke introduced the concept of stereotaxis in 1908.4 Their stereotactic device (the term was modified in the 1970s) was used for animal experimentation only. The first human stereotactic frame used in clinical practice was developed by Spiegel et al. in 1947.5 Though later iterations of the device were invasive,6 the original frame consisted of a plaster cast individually moulded to the shaved head of each patient. A ring adapter was incorporated into this mould and used to affix a modified Horsley–Clarke apparatus (Figure 1).5,7 Air ventriculography (and later, pneumoencephalography) was used for target localization. Its original purpose was to reduce ’emotional reactivity‘ through medial thalamus lesions, thereby replicating the results of frontal lobotomy via ’less drastic‘ means.5 At the time of publication, Spiegel et al. had likely also used the frame to treat various pain and movement disorders and to drain cysts.8 There is no published account of the frame they used for radiotherapy, but it should be noted that Leksell did study under the supervision of Spiegel et al. in Philadelphia, USA.3
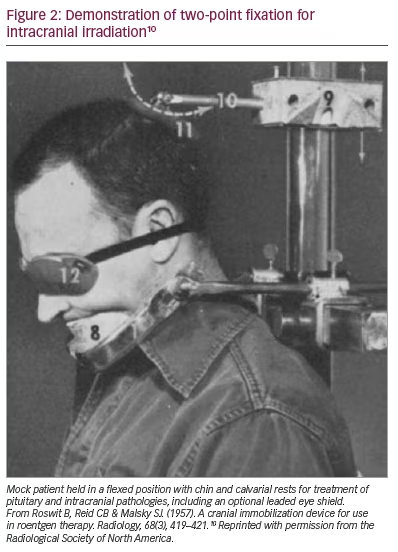
Frames of a different sort were also developed to immobilize the head during radiation therapy of head and neck cancers, into which intracranial pathology was sometimes bundled. The first attempts at stabilization came in the form of two flat wooden pieces placed on either side of the head, thereby reducing rotation but not flexion.9 Bemoaning the lack of commercially available solutions, a fixation device, comprising of brow or chin supports and an articulating calvarial pad, was fabricated by radiologists at the Veterans Administration Hospital in the Bronx, New York, USA.10 Fixation was deemed ’absolute‘. Hundreds of patients were noted to be treated in 2 years preceding publication, although no specifics were given. Figure 2 shows a mock patient in a flexed position deemed ideal for treating pituitary and intracranial lesions.10 A second group, inspired by the apparatus created by noted radiotherapist Dr Isadore Lampe, described a device that paired external immobilization with roentologic confirmation of stable repositioning via the orbitomeatal line.11 Here, too, clinical details are largely absent, but a photograph demonstrating appropriate positioning for posterior fossa irradiation is included. The article concludes, ’Needless to say, the accuracy required for moving field therapy is easily accomplished with this unit’.12 Insofar as the imaging available at the time allowed for accuracy on the centimetre scale (deep cerebral nuclei via pneumoencephalography notwithstanding), this was a true statement.
Computed tomography era
The value of computed tomography (CT) scans in neuroradiology was apparent from its very first clinical application in 1971.13 There were several challenges facing practitioners who wished to integrate this nascent technology into stereotaxy in general, and radiosurgery in particular. One issue was the metallic artifact induced by the commercially available frames of the day. A second concern, more generalizable to diagnostic neuroradiology, was the issue of replicable head placement to facilitate comparisons between temporally separate scans.14 Most descriptions of bringing stereotaxy into the CT era involved invasive frames modified to allow artifact-free imaging.15–22
The earliest attempts to develop a non-invasive solution came from the Karolinska Hospital in Stockholm, Sweden. The very first patient treated on a Gamma Knife (Elekta AB, Stockholm, Sweden) in November 1967 had their head immobilized by a moulded plaster of Paris cast, but there was no attempt to incorporate a stereotactic head frame into the design.23 A non-invasive attempt at true stereotactic irradiation came in the mid 1970s.24 The authors described four goals in developing a fixation device to facilitate stereotactic axial imaging: (1) reduce patient movement to lessen motion artifact; (2) allow reproducible positioning for long procedures; (3) enable precise, transferable geometric target delineation across numerous diagnostic and therapeutic modalities; and (4) facilitate reproducible head positioning for scans performed on different days. Their solution was to use mouldable thermoplast to create a rigid helmet based on bony anatomy. Aluminium hooks were used to attach the helmet to an aluminium-ring base plate that, in turn, could be attached to the orifice of the scanner. The mask could be cut and reapplied on different days. Mean movement was measured at 3 mm, but as much as 13 mm in one case. Error in transforming coordinates between modalities could be reduced by moulding the thermoplast around two or three screws anchored in the calvarium. The authors noted that 50 patients underwent diagnostic imaging using the thermoplast helmet. A modified version, presumably incorporating a stereotactic frame, was noted to have been used for radiosurgical planning to treat three patients with vestibular schwannomas; a separate publication based on these three cases was listed as being submitted for publication but cannot be found in the medical literature (Norén G, Backlund EO, Bergström M, Grietz T: Radiosurgery of acoustic neuromas using stereotaxic computerized tomography).24 The group subsequently modified their design, changing the helmet material for improved comfort and incorporating a mouthpiece to improve accuracy.25,26
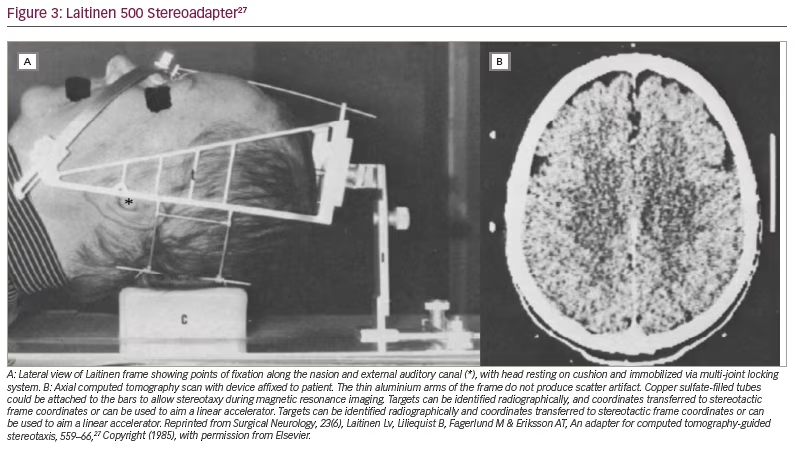
A second non-invasive device, also from Sweden, was introduced in 1985.27 The adapter, eventually referred to by its commercial name (Laitinen Stereoadapter 5000 Frame, Sandstroem Trade and Technology, Welland, Ontario, Canada) (Figure 3),27 had several advantages: it was inexpensive, did not require bespoke helmet fabrication, was sufficiently adjustable to accommodate a wide variety of skull sizes, and allowed reproducible application across sessions. The primary focus of this introductory publication was to tout the frame’s value in ablative functional procedures, but its potential role in facilitating irradiation via linear accelerator was mentioned. Soon after, the frame helped usher in the era of fractionated stereotactic radiosurgery.28,29
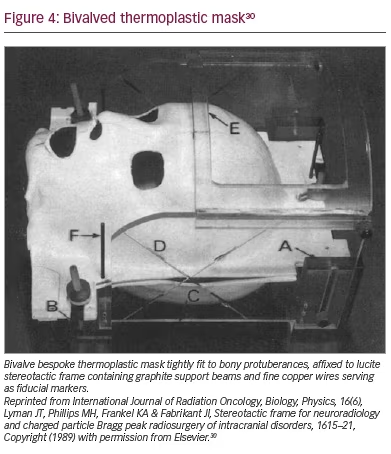
A third apparatus was developed in 1989 at the Lawrence Berkeley Laboratory in Berkeley, California, USA for administration of proton-beam radiosurgery.30 A warmed thermoplastic material was moulded to the front and back of the head to create a removable, bivalve, rigid mask; hair less than one inch in length was necessary to minimize repositioning errors. A stereotactic frame, compatible with CT, magnetic resonance imaging and angiography, was rigidly attached to the mask (Figure 4). Interfacing adapters for diagnostic and therapeutic devices allowed rigid immobilization. Tolerances in the order of 1 mm were reported.30
The era of stereotactic radiotherapy and fractionated radiosurgery
A second wave of innovation came in response to fractionation. As early as the 1920s, it was known that dividing radiation treatments into multiple fractions could induce the desired biologic effects without the toxicity of a single large dose.31 The concept of fractionating targeted radiation in the treatment of intracranial pathology was well established in the early 1990s, but accurate target reacquisition and strict immobilization remained obstacles.32 Some early attempts at administering fractionated radiotherapy involved so-called ’hard docking‘ via invasive frame immobilization, either by leaving the frame on between treatments (as long as 58 days in one study)33 or reapplying it prior to each session.34 For obvious reasons, the hard docking strategy eventually fell out of favour, as wearing an invasive halo-like frame for days or weeks was suboptimal from a patient perspective.
A few intermediate strategies were also trialled. One approach was to improve stereotactic accuracy via scalp implantation of three thin gold wires to serve as radiographic markers; a thermoplastic mask was subsequently used to immobilize during treatment.35 Another was to attach a frame to temporarily exposed transcutaneous screws in a commercially available system (TALON, Nomos Corp., Sewickley, PA, USA).36 The screws could be used to attach a frame for treatment planning and to rigidly affix the head to the linear accelerator. A 6-week treatment paradigm was described, during which three out of nine patients experienced superficial infections and two had one of the screws loosen.36
In contrast, ’soft docking‘ or non-invasive strategies flourished in the 1990s. A number of authors continued to advocate for the fabrication of bespoke helmets using self-hardening bandages.37–39 Heifetz and colleagues developed a series of coordinating head holders for diagnosis (CT, angiography) and radiotherapy.40 The immobilization strategy, via nasion and external acoustic meatus, resembled the Laitinen frame but required three pin tattoos be placed on the forehead/scalp for accurate transition between holders; perhaps because of the need for permanent skin markings, the Heifetz device did not gain widespread acceptance.
For a time, the Gill–Thomas–Cosman frame (GTC, Integra‐Radionics, Burlington, MA, USA) was the most used device for fractionated radiotherapy.41 This frame, developed by two British neurosurgeons and an American physicist, was introduced in 1991.42,43 It consisted of a custom dental mould fixed by two plates and a mouldable occipital pad. Four adjustable straps were used to secure the frame via these fixation points to a Brown–Roberts–Wells base ring (BRW, Integra‐Radionics, Burlington, MA, USA), thereby allowing use of the same imaging and treatment localizers. The straps were typically marked to have the straps tightened to the same length each time. The system afforded an immobilization range of 0.6–1.4 mm in anteroposterior and lateral displacements,43 and its reproducibility across multiweek radiotherapy was found to have an average of 1.1 mm.44 The unit was not without its problems, with the most ire directed at the bite block. In particular, in one study, patients with dentures frequently reported discomfort, with the degree of discomfort being associated with greater within-session motion and need for breaks.45 A group at Harvard Medical School made a number of modifications to the GTC device while preserving its compatibility with the BRW ecosystem, eventually introducing this commercially as the Tarbell–Loeffler–Cosman frame (Integra‐Radionics, Burlington, MA, USA). Out of recognition that anaesthetized paediatric patients could not use the bite block, they offered a glabellar rostral fixation apparatus. Additionally, they created a depth-confirmation helmet that could rigidly attach to the base ring. The helmet contained 25 holes through which graduated probes could be inserted and measurements taken for comparisons with prior sessions. The overall tolerances across multiple fixation sessions averaged less than 1 mm, but there were a few outlying shifts nearing 2 mm.
Incorporation of bite blocks was also trialled. Bite blocks as a means of fixation date back to the early days of CT scans.46 One early example had a custom-moulded bite block manufactured with two cylindrical bolts.47 These bolts served as the only point of fixation to a BRW-like frame that was used for stereotactic localization of a perichiasmatic tumour. Radiosurgery was discussed as a possible application, but not explicitly described.
BrainLab AG had been involved in stereotaxy dating back to the introduction of their invasive head ring. In 1994, the company submitted a German patent for a frameless system, followed by a US patent in 1995,48 and clinical introduction of the system in 1997.49,50 The device consisted of a thermoplastic mask warmed and fitted to each patient (Figure 5).51 A bite block was originally incorporated into the mask in a manner similar to that described by Greitz,25 but it was later rendered optional via a bite block attached directly to the frame. The degree of fixation with the device was also excellent, measuring on the order of 1 mm in multiple planes.51
A novel approach was taken by William Friedman (neurosurgeon), Frank Bova (physicist) and colleagues at the University of Florida, FL, USA.52 The group aspired to decouple immobilization and localization, tasks simultaneously performed by the frames of the day, out of concern that repositioning accuracy could be compromised with dual-function devices. Immobilization came via a thermoplastic mask similar to that used contemporaneously by the CyberKnife (Accuray, Sunnyvale, CA, USA) (see the text below). Localization was conducted with infrared light-emitting diodes affixed to a small lucite plate, in turn rigidly fixed to a custom dental mould. A commercially available stereo camera system, the Optotrak3000 (Northern Digital Inc, Waterloo, Ontario, Canada), was used to triangulate the diodes’ position 100 times per second with sub-millimetre accuracy.52 The entire system was ultimately commercialized by Varian Medical Systems (Palo Alto, CA, USA).
Market pressure
By the late 1990s, the technological innovations necessary for frameless radiosurgery were in place. Neurosurgeons and radiation oncologists, however, were slower to respond. Gamma Knife radiosurgery remained, for the most part, entrenched in the single-encounter surgical paradigm envisioned by Leksell of ’closed-skull destruction of an intracranial target using ionizing radiation‘. Linear accelerator-based stereotactic radiosurgery remained controversial owing to the perceived lack of accuracy compared with Gamma Knife systems.34 The CyberKnife system was, in many ways, the catalyst that triggered the frameless revolution.
The creator of the CyberKnife, John Adler, interrupted his neurosurgical training at the Brigham and Women’s Hospital, Boston, MA, USA to complete a fellowship at the Karolinska Institute, Stockholm, Sweden under Leksell in 1985. During that experience, he began envisioning a frameless system that would both spare the patient the rigors of invasive frame placement and be suitable for use throughout the body.53 The prototype device, the Neutron 1000, entered service at Stanford University, CA, USA in 1994. The US Food and Drug Administration approval for treatment of intracranial tumours came in 1999. Immobilization was via a thermoplastic mesh paired with positional verification via stereoscopic X-rays. It was able to compensate for limited patient movements via an articulating robotic arm. Subsequent models, G2 and G3, brought further advancements in image tracking and accurate delivery.53,54
Procedural and technical comparisons between the Gamma Knife and linear-accelerator radiosurgery were quick to follow,55 but direct comparisons in terms of clinical outcomes analysis trickled through at a much slower rate.56,57 In terms of market share, however, linear-accelerator modalities gained significant ground, undergoing an almost ten-fold increase in the proportion of stereotactic radiosurgery cases between 2003 and 2011.58 The reasons for this shift were multifactorial, but patient comfort related to non-invasive immobilization played an important role.59
Perhaps in response to the commercial success of the CyberKnife, Novalis BrainLab developed a modified version of their aforementioned thermoplastic mask that has been in use since 2006. This design included six optical marking spheres attached to the mask that were used for real-time head tracking, which was found to provide a very low intrafraction error.60 The concept is similar to the aforementioned University of Florida system, but with the bite block omitted.52 The crux of the localization system was light-emitting diodes affixed to a custom dental mould, thereby identifying patient movement. Immobilization was performed by a mouldable thermoplastic mask that did not cover the mouth.60
Other commercial solutions were generalizable across radiosurgery platforms. Li and colleagues, for example, described the combination of the PinPoint (Aktina Medical, Congers, NY, USA) frameless immobilization system with the AlignRT (Vision RT, London, UK) stereo camera-based motion-tracking system.61 The former consists of a custom alpha cradle and a custom bite block rigidly attached to a metal arch affixed to the treatment table. The bite block is fenestrated along its upper surface so a gentle vacuum suction can be applied to the upper palate. The latter uses a total of six ceiling-mounted cameras to monitor the patients’ surface anatomy for movement. The idea of using cameras to track movement and inter-session repositioning is itself an old one, first described in 1978.14
Elekta AB, the company that manufactures the Gamma Knife, assuredly noticed the gradual adoption of non-invasive strategies and data supporting accurate treatment. Their first non-invasive system was the eXtend frame system that incorporated several features described in other systems. Like PinPoint, the vacuum-assisted bite block in combination with a custom occipital head mould limited patient movement. A repositioning check tool, methodologically similar to the depth-confirmation helmet, was used to check for accurate repositioning in fractionated treatments.62 Additional within- (and between-) treatment positioning was checked with cone-beam CT (CBCT), comparing bony anatomy in three dimensions.63
Despite providing excellent immobilization and reproducible head positioning, the eXtend system did not gain widespread adoption.64 Elekta’s second-generation system, Icon, did gain significant traction in the marketplace. This system combines a moulded mesh thermoplastic mask, with integrated CBCT and a high-definition motion-management system that uses an optical marking sphere affixed to the patient’s nose to track movement in relation to spheres affixed to the rigid frame via a stereo camera system.65
The technological advances outlined above, which facilitated widespread adoption of fractionation schemes via non-invasive immobilization, disrupted a brittle equilibrium between neurosurgeons and radiation oncologists. This conflict, encompassing various fronts over many decades, is well reviewed by Giller in his book chapter entitled ‘CyberKnife warfare in America’.66 Giller describes an ‘uncivil’ conflict out of proportion to financial competition alone. At the core of this dispute was tribalism between radiation oncologists and neurosurgeons, each with their own literature, norms and morals. For many neurosurgeons with a clinical interest in radiosurgery, frameless treatment represented an existential crisis, justified by a survey of over 500 radiation oncologists, 20% of whom acknowledged never including neurosurgeons in the planning of stereotactic brain tumour treatment.67
Conclusion
Much like the field of radiotherapy, non-invasive means of facilitating intracranial radiotherapy have evolved significantly over the past 40+ years. We have entered an era in which most patients will not require invasive frame placement. There are many innovations and pressures that collectively led the field here. For the patient, this is assuredly an improvement – frame placement represents a source of significant anxiety and at least some discomfort. For the radiation oncologist, non-invasive frames have opened the possibility of pursuing fractionated treatment plans without sacrificing treatment accuracy. For the neurosurgeon, this era represents both a challenge and an opportunity. Frame placement, typically done by neurosurgeons, guaranteed them a seat at the treatment table. Today’s masks, fabricated and applied by the radiation oncology team, mean neurosurgeons must find other means of bringing value to radiosurgery to justify their continued involvement.
We do believe it is in the patient’s best interest to have a multidisciplinary treatment team involved in treatment planning. In this collaborative environment, both specialties can bring to bear their distinct clinical foci to the patient’s disease. The neurosurgeon has a superior understanding of neuroanatomy and function, allowing for identification of radiosensitive intra-axial structures that can be displaced by mass effect or vasogenic oedema. Additionally, neurosurgeons have expertise in the management of patients with various conditions beyond metastatic cancer, including arteriovenous malformations, meningiomas, and various functional and pain pathologies that can be treated via radiosurgical ablation.
In our institution, we have attempted to facilitate collaboration in multiple ways. Both specialties are represented in weekly collegial tumour boards. Patients are routinely cross-referred between clinics, so the patient has the benefit of discussing treatment options from different clinical perspectives – these discussions frequently, but do not always, concur. Our clinics are co-located in our outpatient cancer centre, requiring only a brief walk from one waiting room to the other. This geographic proximity is additionally beneficial in that the neurosurgical team can discuss and, when necessary, refine the treatment plans formulated by radiation oncologists and radiation physicists. We feel strongly that this multifaceted approach provides the best care possible. We encourage, indeed urge, our colleagues in radiation medicine, neurosurgery and medical physics to continue or, if necessary, adopt some version of this model. This will ensure the continued growth of stereotactic radiosurgery as an increasingly vital part of contemporary medical and surgical care.














