Surgical-site infections remain a significant contributor to hospitalacquired infections despite continued efforts to reduce their occurrence. Infection at the operative site is associated with high morbidity, mortality, and prolonged hospitalization. Typically, in neurosurgical cases the infection rate varies between 1 and 4%. While antibiotic use,1 enhanced patient homeostasis (e.g. with respect to serum glucose levels or body temperature), and wound management are appropriate topics and are analyzed in reviews on the prevention of surgical-site infections,2 pre-operative antisepsis is less frequently considered.3
The rise in antimicrobial resistance makes pre-operative methods to reduce surgical-site infection even more important,4,5 particularly since hospitalized patients tend to have a higher frequency of resistant organisms.6,7 The rise in methicillin-resistant Staphylococcus aureus (MRSA) infections has made antibiotic prophylaxis of this highly virulent organism more difficult.8 This article considers the role of pre-operative antisepsis, which aims to reduce bacterial density in the operative site. The development of a sterile surface concept as part of an approach to reducing surgical-site infection in a neurosurgical setting is also reviewed.
Neurosurgery has several unique features associated with the problem of surgical-site infection: transmissible diseases, hair removal, and indwelling devices. Transmissible diseases, such as spongioform encephalopathy, or transmissible viruses represent a threat to medical staff that is unique to surgery involving the central nervous system. As such, a separate focus on procedures and management of clinical issues, such as blood exposure and sterilization procedures, is required.9 However, the same principles that apply to surgical-site infections generally apply to neurosurgery as well. The major source of infection is endogenous organisms found on the patient’s skin,4,10 and the risk for infection is a balance between patient factors that resist infection and bacterial factors that encourage infection, i.e. bacterial density at the wound site and bacterial virulence.11
However, these factors ignore other influences on infection, e.g. operative time. The relative risk of infection in clean surgeries of less than two hours’ duration has been shown in a recent study to be 12.6%, and this risk doubles to 24.3% in surgeries of more than three hours’ duration. Resistance to infection is further compromised by the use of implanted devices, since it is generally recognized that the presence of a foreign material reduces the host’s capacity to resist pathogens.
Hair removal and its influence on the surgical-site infection rate has been the subject of analysis. While the results are inconclusive in deciding whether hair removal is necessary, it is clear that hair removal with a depilatory agent or with clippers results in a lower surgical-site infection rate than hair removal by shaving.12
The use of known categories of surgical classifications, e.g. ‘clean,’ ‘clean-contaminated,’ ‘contaminated,’ and ‘dirty,’ has long provided a mechanism by which to estimate the risk for infection for a given procedure. However, neurosurgery is sufficiently different that a modified system of infection classification appears justified.13 In patients beyond the neonatal period (where repair of neural tube defects appears to represent a unique category of patient), the presence of implanted synthetic materials supports the separation of this group of patients from ‘clean’ surgical cases due to a significantly higher infection rate.
It has been recognized for decades that reactions to implanted materials within the central nervous system are identical to reactions seen elsewhere in the body, with the addition of gliosis in the central nervous system superimposed on the more typical healing response that leads to fibrosis.14 Similarly, the prevention, diagnosis, and management of infections associated with implanted devices provide challenges similar to those faced with orthopaedic or cardiovascular devices.15 The risk for infection is inversely related to host response, and the ability to resist infection is greatly diminished by the presence of a device.
Both in vitro16,17 and in vivo18 analyses have indicated that the presence of a foreign material results in a localized immune defect that significantly reduces the host’s ability to respond to pathogens. Infection by atypical pathogens of low virulence is commonly associated with immune-compromised patients,19 which further supports the theory of a localized immunologic defect at the site of an implanted device.
As is the case with implanted materials in other sites, the use of prophylactic antibiotics or antibiotic-coated materials has been considered. Analysis of prophylactic antibiotics indicates that protection against infection can be conferred for the 24-hour peri-operative period,20 but is not of any apparent value beyond that time-frame. In a single observational study, there was no benefit with the use of antibiotic-coated devices within the central nervous system.21 However, there has been an association between bacterial density on the skin and subsequent infection of cerebrospinal fluid shunts,22 consistent with the principle that the risk for infection is proportional to wound bacterial content.
By reducing contamination of the wound, 3M™Ioban™2 could reduce the infection rate due to surgeries of long duration, especially if implantation of synthetic materials is to be carried out. Such a reduction would be consistent with published data on the 10-fold reduction in wound contamination in orthopaedic surgery attributed to 3M™Ioban™2 use23— a reduction that can be observed with the use of standard iodine-based pre-operative antisepsis.24
Reduction of skin flora is customarily achieved by the use of broad-spectrum antiseptics. However, the response to antiseptic agents can be highly individual in nature,25 and it is also the case that no antiseptic agent is capable of removing all organisms.3 In the absence of a known ‘minimum’ acceptable density of organisms, a reduction in number of bacteria at the wound site to as low a number as possible is indicated. Since bacterial adherence is a pivotal step in subsequent device-related infection,26–28 providing a sterile surface by using incise drapes also appears beneficial.
Current evidence supports the use of a sterile incise drape with antimicrobial impregnated into the adhesive as a mechanism to reduce the risk for surgical-site infection. Assessment of the efficacy of a subset of currently available surgical incise drapes was carried out in vitro.29 The results are illustrated in Figure 1 for the antimicrobial-impregnated incise drape (3M™Ioban™2).
For the time-dependent in vitro kill rate, expressed in logarithms of colony-forming units (CFU) killed, for an antimicrobial-impregnated incise drape (3M™Ioban™2), values are given as an average ± 1 standard deviation. Staphylococcus aureus and Staphylococcus epidermidis, both methicillin-resistant, are accentuated by enclosure (see Figure 1).
The adhesive surface of the test sample is inoculated with 50μl of a bacterial suspension (containing 5×108 [±0.5 log] CFU/ml) by dispensing 10–12 droplets across the surface. The petri dishes are covered and incubated at 35±2°C for 30 minutes plus one minute; 60 minutes plus two minutes; and 90 minutes plus two minutes (timing starts on contact with the total inoculum volume).
At the appropriate time, the sample is transferred to a blender jar containing 100ml of Difco™ D/E neutralizing broth. Samples are blended for two minutes at low speed. After blending, serial 10-fold dilutions in phosphate-buffered water are plated for each dilution in duplicate, the plates incubated, and colonies counted after 48 hours of culture (72 hours for fungal organisms).30
In a published study on the role of endogenous microflora on neurosurgical-site infections, no relationship was observed between bacterial density before or after skin antisepsis and subsequent infection.31 3M™Ioban™2™ was used in all surgical cases. In a prospective study,32 bacterial densities were measured using 3M™Ioban™2™ without disinfection prior to surgery and compared with bacterial densities with standard antisepsis (Betadine). 3M™Ioban™2™ reduced the bacterial density on the skin, although to a lesser extent than the standard antiseptic agents. The data indicate that 3M™Ioban™2™ reduced the bacterial skin count (measured as CFU) by approximately 0.70 logs. Figure 2 illustrates the relationship between the post-antisepsis and preantisepsis bacterial densities, given as CFU (data courtesy of Dr E Larson).
Six hundred and one patients underwent craniotomy using iodophor antisepsis.31 Fifty-eight patients had only Staphylococcus epidermidis remaining on their skin after antisepsis.
At present, the accepted antisepsis level of a given product is determined by the tentative final monograph on antiseptic products.33 The characteristic behavior of an antiseptic appears as the diagonal line. Every data point below and to the right of the diagonal line indicates that antisepsis was acceptable by current definitions. This situation is illustrated using circles. Failure to achieve acceptable antisepsis is illustrated using squares.
The amount of bacteria necessary to lead to a prosthetic infection can be estimated34–36 at 2 logs (100 CFU), and any value that equals or exceeds this number is illustrated with a filled symbol. As indicated, 22 patients (4%) failed to undergo acceptable antisepsis and 42 patients (7%) had residual bacteria on their skin in excess of 200 CFU. Using the results described, the application of 3M™Ioban™2™ reduces those individuals who have in excess of 2 logs of Staphylococcus epidermidis on their skin to 19 (3% of all patients), effectively reducing those subjects with sufficient organisms to lead to a prosthetic infection by half.
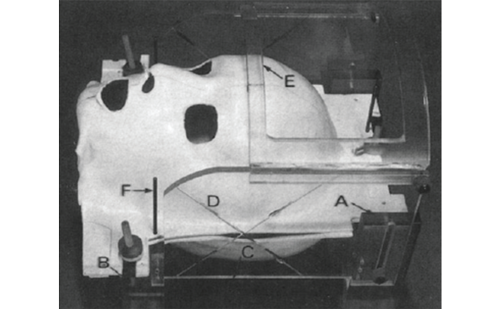
Clinical neurosurgical experience supports the utility of 3M™Ioban™2™. For eight years it has been a standard approach to uniformly use 3M™Ioban™2™ for both spinal surgery and the implantation of electrodes into the subthalamic nucleus for Parkinson’s disease (see Figure 3). In a series of 125 patients given a bilateral implantation for Parkinson’s disease in the subthalamic nucleus, 250 electrodes were implanted and no infections of the intracranial electrodes were noted after a median survey time of more than one year. Given that the duration of the operation was approximately four hours, it is clear that additional protection beyond the use of wide-spectrum antiseptics becomes necessary to maintain a lower risk of surgical-site infection by reducing the cutaneous flora. Similarly, between January and June 2009, 182 patients underwent disc repair, and there has been one case of discitis, yielding an infection rate of 0.54% (GKN, unpublished data).
Conclusion
As with other device-related infections, meticulous surgical methods must be coupled with a process of infection reduction, which can be improved by the production of a sterile surface. The risk for surgical-site infection is proportional to the number of residual bacteria at the wound site, so a reduction in skin bacterial density will be associated with a concomitant reduction in surgical-site infection. In any situation, a randomized prospective clinical study generally carries the highest evidence of proof of efficacy of a given treatment regimen. However, in the presence of low infection rates, sample sizes become too large for such a study and the decision to use a given agent must rest on other information. The cumulative in vitro and in vivo evidence related to wound contamination and extensive clinical experience with implanted neurosurgical devices illustrate the utility of using 3M™Ioban™2 as part of an infection prevention regimen within neurosurgery. ■