Pressure-programmable shunt valves permit non-invasive readjustment of the opening pressure in an implanted shunt to respond to changes in cerebrospinal fluid hydrodynamics.1,5,8,10 Such valves are advantageous in patients with normal-pressure hydrocephalus,1,7–9 arachnoid cyst,9 complications caused by over-drainage of cerebrospinal fluid,5,9 and slit-ventricle syndrome;4,5 however, the magnetically adjustable valve setting may need readjustment after exposure to the intense magnetic fields used in magnetic resonance imaging (MRI). Changes in the valvepressure setting of pressure-programmable shunt valves have occurred after 0.4- and 1.5-tesla MRI.1,3,5–7 MRI units with higherfield strengths of 3.0-tesla are currently under development at many institutions.
The study investigated the effect of exposure to a 3.0- tesla magnetic field on the valvepressure setting of several types of magnetic pressure-programmable valves, which are widely used in the neurosurgery.
Materials and Methods
Valve Characteristics
Five each of the following four types of pressure-programmable shunt valve were tested:
• Sophy Polaris® (Sophysa, Orsay, France);
• Sophy® SM8 (Sophysa);
• Codman-Hakim programmable valve ™ (Codman & Shurtleff, Raynham, Massachusetts, US); and
• Medtronic Strata® (Medtronic, Inc., Minneapolis, US).
The Sophy Polaris is a spring/ball valve. Non-invasive adjustment involves using a magnet to rotate a pressure bar, containing two cobalt-samarium micro-magnets, to change the force exerted on the ball by a semicircular spring attached to the extremities of the bar. The pressure bar is immobilised by a second spring in five positions, corresponding to five pressure settings between 3cmH2O and 20cmH2O. The Sophy Polaris valve has a self-locking system. The immobilising spring is fixed to an H-shaped rotor that can pivot within the body of the valve around the central ruby axis. Two mobile shuttles can slide along the H-shaped rotor. Each shuttle contains a polarised micro-magnet to permanently attract the opposite shuttle. Each shuttle is equipped with a lug, designed to lock the rotorshuttle system in indexing notches, located on the wall of the valve body. The rotor-shuttle system can be unlocked by attracting the shuttles in opposite directions simultaneously. In contrast, a static magnetic field attracts the shuttles in the same direction and the rotorshuttle system cannot be unlocked. The position of the pressure bar can be checked by simple visual inspection after implantation with an external compass or a radiograph.
The Sophy SM8 uses the same system as the Sophy Polaris for changing the pressure setting, but the pressure bar is immobilised by a second spring in eight positions, corresponding to eight pressure settings between 5cmH2O and 17cmH2O. The Sophy SM8 does not have the self-locking system.
The Codman-Hakim programmable valve is a double-ball valve. The inlet valve controls the total pressure of the valve and consists of a synthetic ruby ball held in the valve seat by a flat stainless steel spring. The opening pressure is adjustable between 3cmH2O and 20cmH2O by raising the spring on a spiral polyethersulfone staircase with a ‘stepper motor’ containing a magnet turned by an external electromagnetic field provided by an external programmer. Radiopaque markers disclose the opening pressure setting after implantation.
The Medtronic Strata valve is a spring/ball valve consisting of a ruby ball, a cone valve, a pressure/flow spring and a magnetic rotor. A series of five concentric steps are located at the base of the rotor cassette. The opening pressure is adjustable between 5cmH2O and 25cmH2O by turning the magnetic rotor on the steps of the rotor cassette. The position of the magnetic rotor can be checked in the same way as the Sophy Polaris. A magnet is used to adjust the valve.
Experimental Protocol
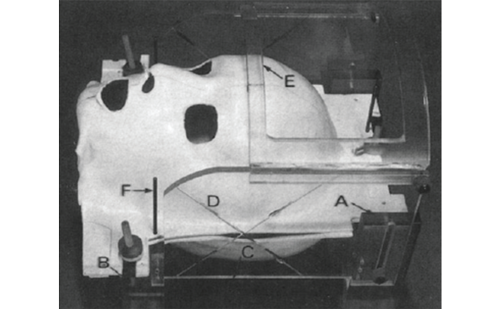
All experiments were performed using a Signa VH/i 3.0-tesla MRI system and a standard head coil. The tests were performed at room temperature. None of the valves was filled with fluid. All four valves were new at the time of testing. The effect of exposure to the static magnetic field was tested. Each valve was taped to the MR phantom, with the long axis of the valve parallel to the main magnetic field. The phantom was then advanced towards the centre of the magnet. A few minutes later, the phantom was removed from the magnet without image acquisition. Changes of the pressure setting were determined through visual inspection with a compass for the Sophy Polaris, Sophy SM8 and Medtronic Strata valves. The pressure setting of the Codman-Hakim valve was confirmed by radiography.
The procedure was repeated three times for each valve at the following pressure settings:
• Sophy Polaris at 3, 11 and 20cmH2O;
• Sophy SM8 at 3, 11 and 20 cmH2O;
• Codman-Hakim at 3, 10 and 20cmH2O; and
• Medtronic Strata at 5, 10 and 15cmH2O.
The changes of pressure setting after exposure to the radiofrequency magnetic field during imaging were tested. Valves showing no changes in pressure setting in the first experimental setup were imaged. The imaging protocol consisted of 3-D spoiled-gradient recalled acquisitions in the three types of images:
• steady-state T1-weighted;
• 2-D fast-spin echo T2– weighted; and
• echo planar diffusion-weighted.
Sequence-specific parameters were as follows:
• For the T1-weighted sequences, repetition time was (TR) 7.8msec, echo time (TE) 1.8msec and field of view (FOV) 200mm (256 matrix).
• For the T2-weighted sequences, TR was 5,000 msec, TE 97.8msec and FOV 240mm (256 matrix).
• For the diffusion-weighted sequences, TR was9,000msec, TE 79.4msec and FOV 240mm (256 matrix).
Each imaging sequence was repeated three times at the low-, middle-, and high-pressure settings. After each sequence, the changes of the pressure setting were determined through visual inspection with compass or radiography.
Statistical Analysis
The number of changes in valve pressure at the two sites from before and after MRI exposure in each valve were analysed using the Mann-Whitney U-test. A probability value of less than 0.05 was considered statistically significant.
Results
Table 1 summarises the data obtained with the four types of programmable valves after exposure to the static magnetic field. The pressure setting of the Sophy Polaris was unchanged at all pressure settings in all three tests (p<0.001). The pressure setting of the Sophy SM8 was unchanged at 3cmH2O but was changed from 11cmH2O or 20cmH2O to 3cmH2O in all three tests. The pressure setting of the Codman- Hakim valve was unchanged at 10cmH2O but was changed from 3cmH2O or 20cmH2O to 10cmH2O or 3cmH2O in all three tests. The pressure setting of the Medtronic Strata was unchanged at 10cmH2O but was changed from 5cmH2O or 15cmH2O to 10cmH2O in all three tests. The second experiment was performed with the Sophy Polaris only. All pressure settings studied were unchanged after T1-, T2-, and diffusion-weighted MRI.
Discussion
This study tested the compatibility of four types of pressure-programmable valves with a 3.0-tesla magnetic field, and demonstrated that only the Sophy Polaris maintained its pressure settings after exposure to the 3.0-tesla static and radiofrequency magnetic fields.
The static magnetic field caused changes in the pressure settings of the Sophy SM8, Codman-Hakim, and Medtronic Strata valves. The pressure setting of the Sophy SM8 was changed to 3cmH2O, the lowest pressure of the valve, in all three tests. The pressure setting of the Codman-Hakim and Medtronic Strata valves were changed to 10cmH2O, the middle pressure of these valves, in all three tests except one test of Codman-Hakim. These findings suggest that the direction and degree of change is predictable in a 3.0- tesla magnetic field. In contrast, several investigators have reported that changes in the pressure setting were not predictable after exposure to magnetic fields of 0.4, 1.5 and 3.7teslas. The difference between the previous and the present results may depend on the strength of the static magnetic field.
The pressure setting did not change in the Sophy Polaris under any conditions studied. This valve has a self-locking system that presumably prevented any change in the pressure setting during 3.0-tesla MRI. Changes in the intracranial pressure may affect patients during and after MRI. Patients with slit-ventricle syndrome can experience symptoms such as headache or loss of consciousness within a few hours.2
Therefore, the Sophy Polaris, in which the pressure setting does not change during 3.0-tesla MRI, may benefit such patients. The pressure setting should still be checked after exposure to the intense magnetic field of the MR imager, however, because the number of tested valves and the number of tests are too small to ensure stability.
Conclusions
This study demonstrated that only the Sophy Polaris valve retained the pressure settings after exposure to 3.0-tesla static and radiofrequency magnetic fields. Although a patient with any of the tested valves can undergo MRI, the patient with a Polaris valve is less likely to require adjustment after the procedure. This valve may be beneficial for shunt-dependent patients who need a programmable valve and will undergo 3.0-tesla MRI. ■
This article is a reprint from The Journal of Neurosurgery: Pediatrics (August 2005);103: pp.163–165.