Gastroesophageal reflux disease (GERD) is a chronic condition affecting over 750 million people globally.1 The disease causes acid from the stomach to reflux into the oesophagus, leading to epigastric discomfort, cough, sore throat and chest pain.1 Left untreated, this disease can lead to oesophagitis, gastritis, oesophageal metaplasia, gastrointestinal ulcers, strictures and bleeding.2 For mild cases of acid disorders, management includes a combination of dietary modification, antacids and histamine type 2 receptor blockers (H2RAs).3 Both antacids and H2RAs transiently relieve symptoms but do not prevent chronic stomach acid-induced damage to the gastrointestinal tract.3 For moderate-to-severe cases of GERD, the primary medical treatment is proton pump inhibitors (PPIs). PPIs irreversibly inhibit gastric hydrogen potassium adenosine triphosphatase pumps and are available on prescription and over the counter.4 PPIs have increased significantly in recent years, especially in older adults.5,6 One study found a threefold increase in PPI prescriptions between 2002 and 2009, with almost 10% of ambulatory patients receiving a PPI prescription.7 However, PPI use can be associated with adverse effects, including osteoporosis, Clostridium difficile infection, anaemia and electrolyte abnormalities.8 In addition, it has been suggested that there may be a risk of cognitive decline with long-term PPI use; however, this suggestion is highly controversial.9–11
Recent studies have suggested that long-term PPI use may adversely affect cognition.12,13 The first major study to report an association was a prospective cohort study that recruited and followed 3,327 subjects age ≥75 years without dementia for 6 years.14 The study demonstrated that the use of PPIs was associated with a significantly increased risk of dementia. Since then, there have been several studies with conflicting conclusions regarding the use of PPIs and cognitive decline.15–17
Several mechanisms have been proposed to explain the association between PPIs and dementia. Alzheimer’s disease (AD), the most common type of dementia, is characterized by two histological findings: The accumulation of amyloid beta peptide (Aβ) plaques and neurofibrillary tangles composed of hyperphosphorylated tau protein.18 Studies have shown that PPIs can cross the blood–brain barrier, increase Aβ levels and interact with tau protein,19,20 providing cellular and molecular evidence that supports the possible impact of PPIs on cognition. The purpose of this paper is to perform a systematic review on the available literature regarding the potential risk of PPI use in older adults in relation to cognition. We also review the proposed pathophysiological mechanisms of potential cognitive impairment induced by PPI use. We discuss the strengths and limitations of the existing evidence and offer guidance on best practice and improved study design.
Methodology
A systematic review was performed by searching PubMed for studies published between January 2015 and May 2022. Cohort studies and randomized controlled trials (RCTs) that examined the risk of dementia or AD associated with PPI use were included. The following key words were searched: “proton pump inhibitors”, “PPI”, “lansoprazole”, “omeprazole”, “esomeprazole”, “pantoprazole”, “Protonix” OR “omeprazole”; AND “dementia”, “Alzheimer disease”, “cognitive decline” OR “cognitive impairment”; AND “randomized controlled trial”, “prospective studies” OR “cohort studies”. Studies were considered eligible if they met the following criteria: PPI use occurred once only in patients included in the study and dementia or AD represented one outcome of interest. Studies were excluded if the full text or data were not available. Reviews, commentaries and letters were also excluded.
Results
Our review included 17 studies in total: two were cross-sectional studies, one was a case-control study, 12 were cohort studies and two were RCTs. PPI use was associated with an increased risk of dementia in six studies. No association between PPI use and the risk of dementia was shown in eight studies and two studies showed a decreased risk of dementia with PPI use. There was no association between PPI use and AD in one study, but the same study showed that PPI use is associated with an increased risk of non-AD dementia. A summary of the studies included is presented in Table 1.14–17,21–33
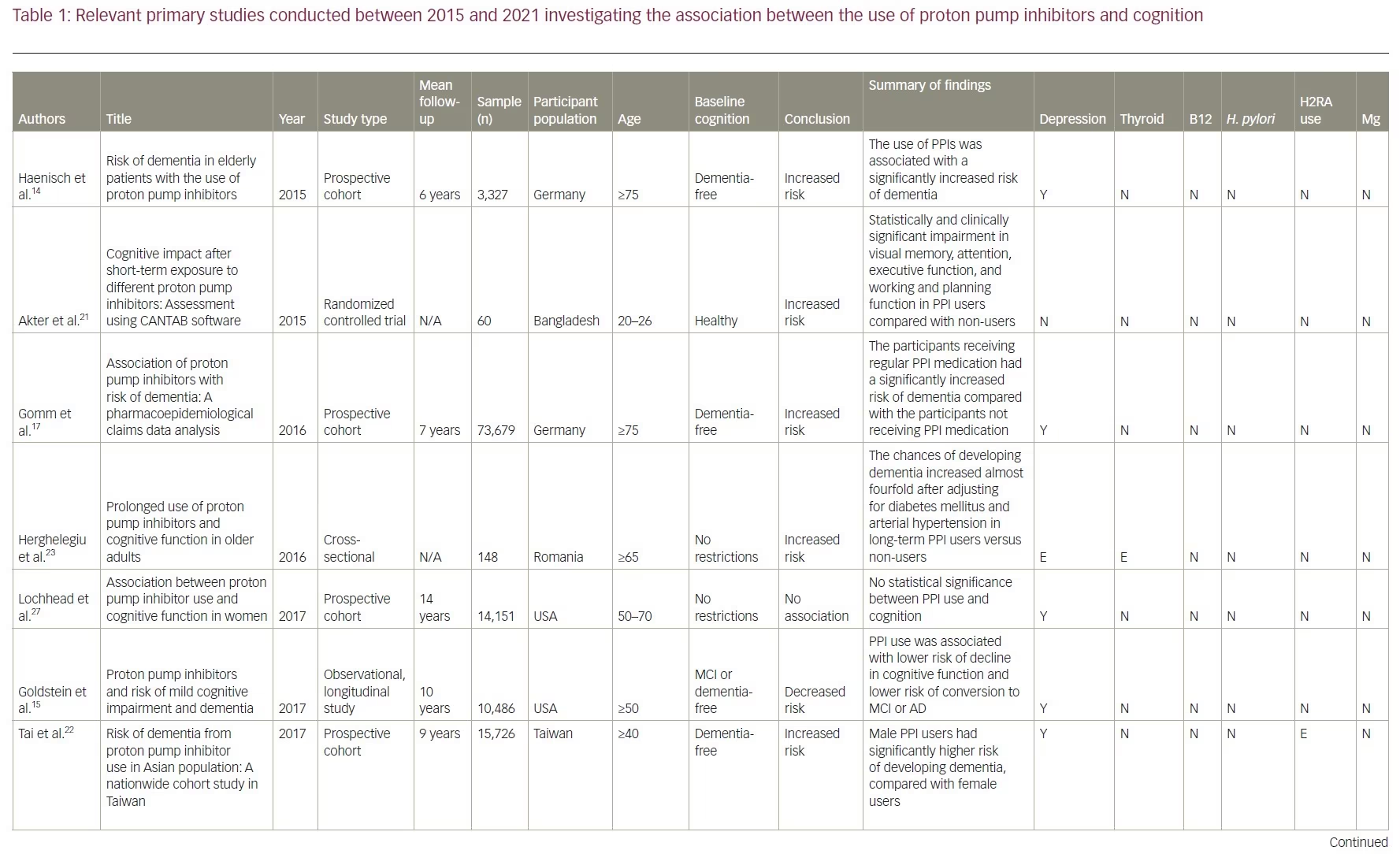
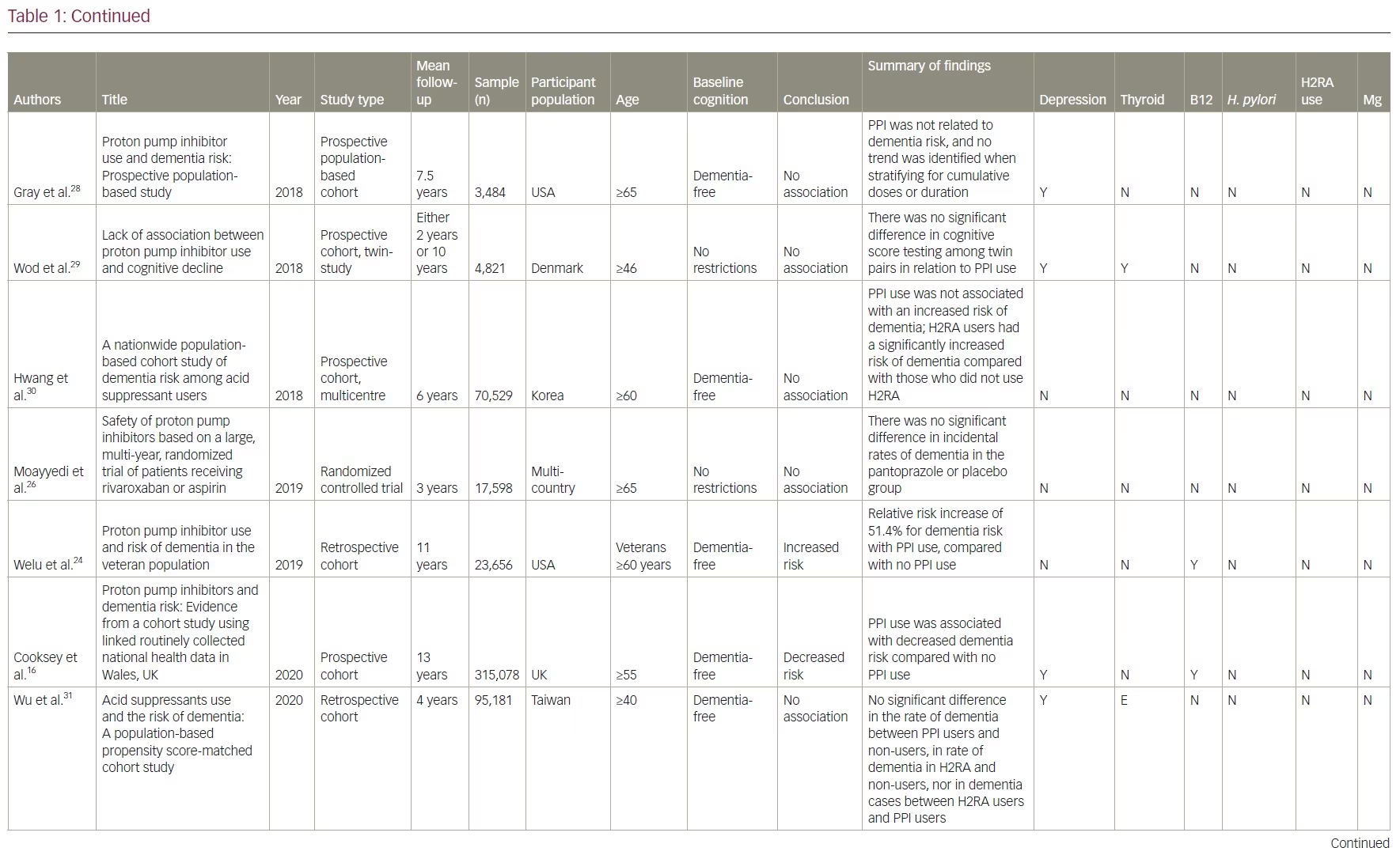
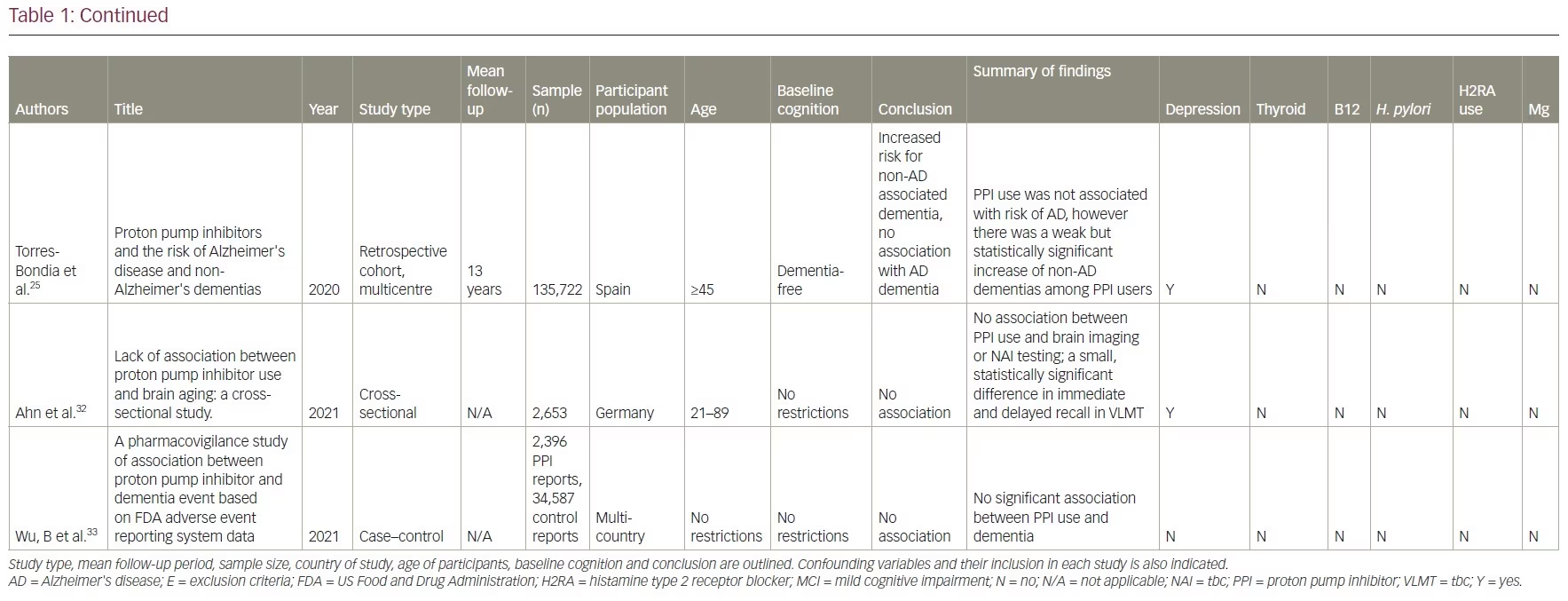
Studies supporting a link between proton pump inhibitor use and dementia
Two studies conducted in Germany first brought attention to a link between PPI use and dementia.14,17 The first was an epidemiological study published in 2015.14 In it, 3,327 subjects aged ≥75 years without a dementia diagnosis were recruited and followed up for 6 years.14 The use of PPIs was associated with a significantly increased risk of all-cause dementia (hazard ratio [HR] 1.38, 95% confidence interval [CI] 1.04–1.83) and AD (HR 1.44, 95% CI 1.01–2.06).14 The second was a prospective cohort study published in 2016.17 Using a sample of older patients from the German statutory health insurer, Allgemeine Ortskrankenkassen, this study included 73,679 patients aged ≥75 years without a pre-existing dementia diagnosis. The study followed patients from 2004 to 2011, with cognitive assessments taking place every 18 months. This study showed similar results to the first one: the patients receiving regular PPI medication had a significantly increased risk of incident dementia compared with those not receiving PPI medication (HR 1.44, 95% CI 1.36–1.52; p<0.001). Owing to its large sample size and long duration, this study was among the first to bring attention to the possible adverse effects of PPIs in older patients. It also considered confounding factors and found that older age, depression, stroke and diabetes also increase the risk of dementia but to a lesser degree when compared with PPI use.
Furthermore, a 2015 RCT concluded that five PPIs (i.e. omeprazole, lansoprazole, rabeprazole, pantoprazole and esomeprazole) had a negative impact on cognition.21 In the trial, 60 participants aged between 20 and 26 years were recruited and randomized into six groups. Each group contained 10 individuals who received either one of the five types of PPI or placebo for 7 days. At the end of the study, the patients were assessed using the Cambridge Neuropsychological Test Automated Battery which includes visual memory tests, executive function tests and attention tests. The results showed significant impairment in visual memory, attention, executive function, and working and planning function in participants taking any PPI. The study did not analyse data based on participants’ sex, age, comorbidities or apolipoprotein E4 (ApoE4) allele, which are all potential confounding factors.
In 2017, a cohort study in Taiwan recruited 15,726 participants aged ≥40 years who were free of dementia at baseline and found that PPI users (n=7,863; average follow-up 8.44 years) had a significantly higher risk of dementia than non-PPI users (n=7,863; average follow-up 9.55 years) (adjusted HR [aHR] 1.22, 95% CI 1.05–1.42; p=0.009).22 Subgroup analysis showed a higher incidence of dementia in PPI users diagnosed with depression (aHR 2.73, 95% CI 1.91–3.89; p<0.001), hyperlipidaemia (aHR 1.81; 95% CI 1.38–2.38; p<0.001), ischaemic heart disease (aHR 1.55, 95% CI 1.12–2.14; p=0.008) and hypertension (aHR 1.54; 95% CI 1.21–1.95; p<0.001).22 They also found that men using a PPI had a significantly higher risk of developing dementia (HR 1.24, 95% CI 1.01–1.52; p=0.040) than women (HR 1.21, 95% CI 0.97–1.50).22 These findings reflect with the results of the study by Gomm et al., which also found that the occurrence of incident dementia with PPIs was slightly more pronounced in men (HR 1.52, 95% CI 1.33–1.74) than in women (HR 1.42, 95% CI 1.33–1.51).17
Two of the selected studies analysed the risk of dementia associated with different types of PPI.17,22 The first found an elevated risk of dementia with omeprazole (HR 1.30, 95% CI 1.09–1.54; p=0.003) but not with pantoprazole (HR 1.36, 95% CI 0.98–1.89; p=0.067) and lansoprazole (HR 1.20, 95% CI 0.98–1.46; p=0.080).22 The second study detected an elevated risk of dementia for omeprazole (HR 1.51, 95% CI 1.40–1.64; p<0.001), pantoprazole (HR 1.58, 95% CI 1.40–1.79; p<0.001) and esomeprazole (HR 2.12, 95% CI 1.82–2.47; p<0.001).17
These studies collected data from national databases or national insurers and thus have heterogeneous diagnostic methods that are affected by individual diagnosticians’ expertise. To minimize the impact of this variability, a Romanian study recruited patients from the Ana Aslan’ National Institute of Gerontology and Geriatrics in Bucharest.23 They included 148 patients aged >65 years, 74 of which were long-term PPI users and 74 controls. Long-term users of PPIs were defined as those undergoing more than 6 months of continuous or nearly continuous administration of any PPI per year for the previous 3 years or more.23 All patients were evaluated with the Mini Mental State Examination and the Clock Drawing Test. The study found that the odds of developing dementia, after adjusting for diabetes mellitus and hypertension, increased almost fourfold in long-term PPI users (odds ratio [OR] 3.67, 95% CI 2.23–19.15; p=0.002).
A retrospective cohort study published in 2019 looked at data extracted from the US Department of Veterans Affairs electronic health records between 2005 and 2015.24 Records of 23,656 veterans were included in the study, with 11,828 veterans in both the PPI cohort and the non-PPI cohort. In the PPI group, 9.5% of veterans developed dementia compared with only 6.3% of veterans in the non-PPI group. The study found an increased risk of dementia in PPI users versus non-users (OR 1.55; p<0.001) and a relative risk increase of 51.4% for dementia risk with PPI use compared with no PPI use. This study also found that B12 levels in patients with dementia were higher than in those not diagnosed with dementia, indicating that PPI use may not be associated with B12 deficiency.
A retrospective cohort study was conducted using data from January 2002 to December 2015 in the Catalan health service system.25 It included PPI users (n=36,360) and non-users (n=99,362). A lag window of 5 years was considered between the beginning of the PPI treatment and the diagnosis of dementia. This study found that PPI use was not associated with risk of AD (adjusted OR 1.06, 95% CI 0.93–1.21; p=0.408). However, a weak but significantly increased risk of non-AD dementia was observed among PPI users (adjusted OR 1.20, 95% CI 1.05–1.37; p=0.007).
Studies demonstrating a lack of an association between proton pump inhibitor use and dementia
One double-blind RCT assigned 17,598 participants aged ≥65 years with stable cardiovascular or peripheral vascular disease to either pantoprazole 40 mg daily or placebo for 3 years.26 Participants were interviewed every 6 months about the incidence of dementia and other potential treatment associated risks. The study found no significant differences in the incidence of dementia between groups.26 The study was limited as no cognitive testing or neurological screening was conducted. In addition, the randomization was limited by a large subgroup of almost 10,000 participants who required long-term PPI therapy or refused to discontinue pre-existing acid-suppression therapy.
There are four prospective cohort studies and one retrospective cohort study from North America, Asia, and Europe with a follow-up duration of between 2 and 10 years that demonstrated no association between the use of PPIs and dementia.27–31 In one cohort study, researchers identified 283 twin pairs that differed in their use of PPI using the Longitudinal Study of Aging Danish Twins (LSADT) (median age 75) and the Middle-Aged Danish Twin study (MADT) (median age 58).29 LSADT participants were followed for 2 years, and MADT participants were followed for 10 years. PPI use was calculated using prescription register data. The study tested participants at baseline and follow-up through multi-component testing, which included fluency, forward digit span, backward digit span, and immediate and delayed recall. They found no significant difference in cognitive testing scores among these twin pairs.
Another cross-sectional study found no significant differences in brain magnetic resonance imaging (MRI) between PPI users and non-users.32 This study used survey data from the Study of Health in Pomerania in Germany and identified 2,653 participants aged 21–89 who underwent a brain MRI. These participants were divided according to their PPI intake, which was based on participants interviews, and cognition was tested using the Verbal Learning and Memory Test and the Nuremberg Age Inventory. These results were deemed not clinically significant since the cognitive decline in the PPI group was less than the expected cognitive decline with increased age (mean age in PPI cohort was 60 versus 51 in the non-PPI cohort). However, these results were deemed not clinically significant, as it was less than the same change associated with increased age (mean age in PPI cohort was 60 versus 51 in the non-PPI cohort).
A multinational case-controlled study was conducted investigating US Food and Drug Administration adverse event claims about PPI use and dementia.33 They included 2,396 cases of dementia event reports related to PPI use by health professionals. This study used US Food and Drug Administration reports of dementia associated with 24,920 anticholinergic drug cases and 9,667 benzodiazepine drug cases as positive controls. They found no significant association between PPI use and dementia (proportional reporting ratio [PRR] 0.98, 95% CI 0.94–1.02), while the positive controls did show an association with anticholinergics (PRR 2.84, 95% CI 2.81–2.88) and benzodiazepine (PRR 3.52, 95% CI 3.46–3.58). The strengths of this study included a large sample size with multiple countries reporting, the investigation of six PPIs and strong positive controls. Limitations included potential selection bias in adverse event reporting and no verification of the actual dementia diagnoses.
Studies suggesting that proton pump inhibitor use decreases the risk of dementia
There are two prospective, cohort studies that show a decreased risk of dementia with PPI use.15,16 One used National Alzheimer’s Coordinating Center data from between 2005 and 2015 and followed adults aged ≥75 years with either normal cognition (n=7,404) or mild cognitive impairment (MCI) (n=3,082) at baseline for 10 years.15 The National Alzheimer’s Coordinating Center database diagnoses of neurological status were based on combined neuropsychological testing and clinical dementia rating scores. Participants were placed into one of three groups based on their report: those taking PPI at every visit, those taking them intermittently and those who had never taken them. They found that subjects with normal cognition at baseline who used PPIs demonstrated a lower risk of cognitive decline compared with individuals who did not take PPIs (HR 0.73, 95% CI 0.55–0.97; p=0.03). They also found that subjects with baseline MCI had a reduced relative risk of conversion to dementia with intermittent PPI use (HR 0.86, 95% CI 0.76–0.98; p=0.03), and there was no significant association with consistent PPI use (HR 0.83, 95% CI 0.67–1.02; p=0.08).
The other study used information from the Secure Anonymised Information Linkage Databank in Wales, UK, and identified 183,968 participants with a history of PPI prescription and 131,110 without PPI exposure.16 Participants were aged ≥55 years, and those with MCI or dementia were excluded. The study followed subjects for an average of 13.04 years and found that PPI use was associated with decreased dementia risk (HR 0.67, 95% CI 0.65–0.67; p<0.01). As these two are cohort studies, there may be significant differences between the twin pair in cognitive performance regarding access to healthcare that may confound the documented prescription of PPIs. In particular, the second study showed a significantly higher rate of death in non-PPI exposed groups compared with the PPI-exposed group (difference 9.8%, 95% CI 9.5–10.2). This differential rate of death among participants and possibly poorer health outcomes may have confounded the results.
Discussion
The studies included in our review investigated the association between PPI use and cognitive decline using large sample sizes combined from different populations worldwide. They showed conflicting results, meaning that the extent of the impact of PPIs on cognition is still disputed.
Explanatory mechanisms
Several mechanisms have been proposed to explain the association between PPIs and dementia. One mechanism involves Aβ, a cardinal feature of AD pathology.18 Lansoprazole, a PPI, was shown to enhance Aβ production and inhibit Aβ degradation in cell and animal models.13 PPIs can inhibit proton pumps in lysosomes, which create an acidic environment that allows the degradation of Aβ.34,35 By inhibiting these pumps, PPIs inhibit the degradation of Aβ.34 Another proposed mechanism involves the tau protein, which plays an important role in stabilizing microtubules in neurons.36 Hyperphosphorylated tau form neurofibrillary tangles, which is a hallmark of AD.37 Studies showed that the benzimidazole ring in lansoprazole has a high affinity with the core protein of tau.38,39 However, no research has shown whether this interaction causes changes in the tau structure or prevents tau from stabilizing microtubules.
Other studies have shown that PPIs exert anti-inflammatory effects.40 By activating the signal transducer and activator of transcription 3, lansoprazole and omeprazole decrease interferon-γ-induced astrocytic neurotoxicity.40 Furthermore, a previous study had identified lansoprazole as a nuclear liver X receptor agonist, which increases apolipoprotein ApoE levels in primary astrocytes.41 ApoE complexes inhibit Aβ aggregation, thereby supporting the theory that lansoprazole has a neuroprotective effect.42 More studies are needed to understand such molecular interactions and their impact on cellular function.
Limitations of the studies
Identification of cognitive decline
The studies considered in this paper have a few limitations. First, these studies adopted different criteria for diagnosing dementia. Some studies required stringent diagnostic tests, some relied on reviews of medical records, while others used participant self-reports. For example, one study classified veterans as having dementia if they received a diagnosis of dementia based on International Classification of Diseases codes or prescription records of cholinesterase inhibitors, memantine, or ergoloid mesylates.24 Another study required multiple documented visits and a prescription for cholinesterase inhibitors.30 This classification relies on the clinicians’ willingness to prescribe anti-dementia medications, which can be highly variable. It also does not address whether all clinicians use the same standardized neurocognitive tests to make the dementia diagnosis, or whether the diagnosis is made by an experienced neurologist or geriatric psychiatrist according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria. Four studies used stringent diagnostic criteria.14,22,31,32 Tai et al. and Wu et al. specified that the diagnoses were made by a neurologist or psychiatrist.22,31 One study required rigorous neurocognitive testing, including the Mini Mental State Examination, the assessment of activities of daily living and the Hachinski–Rosen Scale.14 Another study conducted its own cognitive testing using the Nuremberg Age Inventory, the Verbal Learning and Memory Test, and brain MRI.32 However, other studies did not require stringent diagnostic criteria since the diagnostic process is difficult to control in retrospective chart review studies.
Second, many studies do not differentiate dementia between subtypes in their analyses. One study considered the diagnosis of dementia to be valid if it was one of the following: unspecified dementia only, AD only, vascular dementia only, delirium with dementia only, senile degeneration only and a mix of the above.17 More than 80% of patients with a diagnosis of dementia in this study received either unspecified dementia or mixed dementia diagnoses. Although one study showed no difference in findings when different types of dementia were considered,14 another study found that the incidence of AD was not higher among PPI users but that there was a slight increase in non-AD dementias, indicating that PPI use may increase the risk of certain but not all types of dementia.25 By grouping all-cause dementia as the outcome, the studies could be reducing the power of the current data in showing a statistically and clinically significant correlation of PPI use to a specific type of dementia. Examining the association between PPI use and different subtypes of dementia would better elucidate if and how PPIs impact cognition. The variations in the diagnostic criteria of dementia between the studies included underline the perplexity of pathologies in older adults and raise a challenge in the investigation of PPIs and cognition.
Third, most studies did not account for a lag time between the start of PPI usage and the onset of dementia. A few exceptions include Torres-Bondia, who accounted for a lag time of 5 years,25 and Hwang et al., who excluded participants diagnosed with dementia within the first 3 years of the study.30 However, both of these time frames of exclusions are arbitrary. It is unclear how long it takes for PPIs to have an impact on cognition, assuming there is one. AD, as the most common type of dementia, progresses slowly over the course of several years.18 The latency of AD requires a long follow-up. Some cohort studies have follow-up durations as short as 2 years, which may be insufficient to account for lag time.29 However, without understanding the mechanisms, the lag time would still be arbitrary.
Multiple confounding factors
Another limitation that applies to most studies is the presence of confounding factors. Although most of the studies controlled for diabetes, hypertension, sex and age, there are countless other possible confounding factors, ranging from socioeconomic status and diet to B12 level, Heliobacter pylori (H. pylori), depression and thyroid deficiency. These factors were not included in most studies. One study showed an increased risk of dementia in people with GERD after adjusting for PPI use, indicating a direct link between these two comorbidities.43 Poor social engagement and loneliness are also associated with an increased risk of dementia,44 and mental factors also play an important role in the development of GERD.45 Not accounting for multiple confounding factors adds uncertainty to many of the clinical studies looking for a link between PPI use and cognitive impairment.
It is well accepted that low vitamin B12 levels are associated with cognitive deficits.46 A case-controlled study showed that gastric acid inhibitor use is significantly associated with lowering B12 levels.12 PPIs lower gastric acidity, which is necessary for the absorption of B12, which in turn increases the risk of cognitive impairment.12 In fact, many researchers propose that PPIs increase the risk of dementia by causing B12 deficiency.47 Only two studies mentioned above included B12 deficiency as a factor.16,24 Welu et al. showed that B12 levels in the dementia group were significantly higher than in the dementia-free group,24 indicating that dementia is not associated with low B12. However, they were unable to obtain information on whether certain patients were taking B12 supplementation to treat previous B12 deficiency. When B12 deficiency was removed from the model in the study by Cooksey et al, it showed a benefit of PPI use relative to cognition.16
Another confounding factor that the studies did not consider is H. pylori infection, which has been shown to cause B12 deficiency and presents as epigastric pain and heartburn, similar to GERD.20 One study showed undiagnosed H. pylori infection rates in Canada to be 21%, with the lowest rate being among people born in South and Central America (5.6%) and the highest among people of African descent (71.4%).48 Due to the similar clinical presentations between H. pylori infection and GERD, undiagnosed H. pylori infection is often treated with long-term PPI use. Many people take PPIs long-term without undergoing a gastroenterology workup.23 Moreover, some studies have shown an increased risk of dementia with H. pylori infection.49 Considering the possible large numbers of undiagnosed H. pylori infections in patients receiving PPI treatments, H. pylori status should be closely examined before an association between dementia and PPI use is considered.
Depression is another possible confounding factor. Depression and cognitive disorders, including dementia and MCI, are common in old age.50 Depression in older adults often presents similarly to dementia and is associated with a two- to fivefold increased risk of dementia.50,51 Studies show that depression may be both a risk factor for and a prodrome of dementia. In addition, the use of PPIs was associated with an increased adjusted probability of depression (OR 2.38, 95% CI 1.02–5.58; p=0.045), and higher PPI dosages were associated with an increased probability of depression (p for trend = 0.014).52 Twelve out of 17 studies reviewed considered depression as a covariate, but only one study excluded patients with depression.23 Five studies found that people with regular PPI usage were more likely to have depression.14-17,22 For example, in one study, 32.7% of patients with PPI use had depression, while only 20.1% of patients without PPI use had depression.16 Many factors can contribute to the concurrence of PPI use and depression, including increased age, elevated body mass index, smoking, anxiety and decreased physical activity.53 Depression is a confounding factor that needs to be considered when investigating the relationship between PPI use and dementia.
Disorders of the thyroid gland, particularly hypothyroidism, can increase the risk of dementia and complicate diagnosis.54 Studies have shown that dementia risk increases with thyroid stimulating hormone, a laboratory indication for hypothyroidism.54 The symptoms of hypothyroidism include lethargy, cognitive impairment, low mood and psychomotor retardation, which can appear clinically similar to dementia.55 Moreover, both overactive and underactive thyroid disorders can cause gastrointestinal symptoms such as dysphagia, epigastric pain and symptoms associated with gastrointestinal dysmotility, which may lead to a prescription for PPIs.56 Most studies did not account for thyroid disorders, except for two studies that excluded participants with prior diagnoses of hypothyroidism23,31 and one study that adjusted for thyroid disorders.29
One of the known side effects of PPI use is an increased risk of electrolyte disturbances, particularly hypomagnesemia.57 However, electrolyte imbalances, including of magnesium, were not controlled for in the study analyses. Hypomagnesemia is associated with a higher incidence of dementia; one study showed a 24% higher rate of incident dementia in those having the lowest quintile of serum magnesium.58 In clinical practice, the measurement of magnesium is excluded from the standard complete metabolic panel and requires a separate order. Therefore, incidences of electrolyte disturbances with PPI use may be missed. Another confounding factor is the use of H2RAs. One study showed that H2RA users had a significantly increased risk of dementia compared with non-H2RA users (HR 1.31, 95% CI 1.13–1.51), whereas PPI use was not associated with increased risk.30 Another study found that H2RA users had an increased risk of earlier development of dementia relative to PPI users.59 Thus, the use of H2RA may be associated with an increased risk of dementia independent of PPIs. However, many studies did not control or screen for H2RA use. One study excluded patients who were currently or previously regularly using H2RAs (more than 3 months in a year),22 but the other studies did not control the use of H2RAs as a confounding factor.
Furthermore, these studies did not explore the influence of diet on PPI use and dementia. A study on the diet of patients with GERD found that patients are less likely to have citrus and alcohol in their diet and more likely to consume soft drinks, tea and fatty foods.60 Moreover, there is increasing evidence that a healthy, well-balanced diet may be protective against cognitive decline, while unhealthy diets can worsen cognition.61 Taken together, a difference in dietary composition between PPI users and non-users may also be confounding the results.
Limitations of our study
The limitations of our study include the heterogeneity of the research studies included. This heterogeneity is due to a lack of homogenous research design and diagnostic guidelines across countries and institutions. Due to the scarcity of RCTs in this area, we also included one RCT conducted on young adults. Large, longitudinal, randomized, placebo-controlled studies controlling for variables noted in our paper are needed before definitive conclusions can be reached on the relationship between PPI use and the risk of dementia.
Conclusions
Studies have demonstrated conflicting results regarding the association between long-term PPI use and cognitive decline, and the extent of the impact of PPIs on cognitive functions remains controversial. Despite a variety of studies and sample sizes, the overall quality of evidence for PPIs adverse effects on cognition is low. Baseline differences between PPI users and non-users and multiple confounding factors make studying the potential impact of PPI use on cognitive decline challenging. While there is not enough solid evidence showing that PPIs are associated with worsening cognition, there are still several risks associated with the prescription of PPIs in older adults.62–64 Nonetheless, GERD can significantly impact quality of life, and untreated disease can lead to serious complications. The best recommendation is to prescribe PPIs judiciously, prioritizing lifestyle and dietary changes for controlling symptoms, and sharing decision-making with patients and care partners. Future studies should take into account confounding factors and have a stringent study design, including the patient inclusion and exclusion criteria and study duration. More high-quality, long-term, controlled studies and investigations of purported pathophysiological mechanisms are needed.














