Healthcare workers are currently facing the coronavirus disease 2019 (COVID-19) pandemic and its complications, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Patients usually suffer from respiratory problems and non-specific neurological symptoms, such as headache, dizziness and ataxia, as well as specific neurological complications in some patients.1,2 Acute respiratory distress syndrome is a life-threatening condition that can be caused by SARS-CoV-2. This abnormal inflammatory condition can cause thrombotic/thromboembolic complications.3,4 This case report highlights that thrombotic/thromboembolic complications may occur in young individuals without significant medical history.
Case presentation
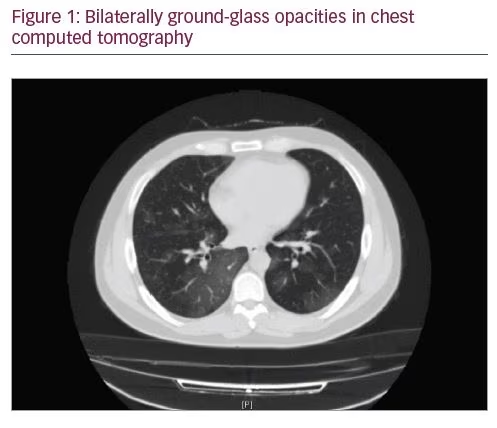
A 33-year-old male patient presented to the emergency department of our hospital on 31 March 2020, with complaints of a high fever and cough. His body temperature was 39.1°C. He had normal neurological findings, and there was no relevant past medical or family history. The results of his blood tests revealed leukocytosis (11.6 x 10³/µL [4.06–10.6 x 103/µL]), lymphopaenia (0.8 x 10³/µL [1.9–7.0 x 10³/µL]), and high C-reactive protein level (15.9 mg/dL [<0.5 mg/dL]). His coagulation parameters were as follows: thrombocytes: 178 x 103/µL (150–439 103/µL); D-dimer: 0.61 mg/L (<0.5 mg/L); fibrinogen: 444 mg/dL (150–400 mg/dL); and lactate dehydrogenase: 341 IU/L (85–227 IU/L). His chest computed tomography (CT) showed bilaterally ground-glass opacities (Figure 1). Other laboratory tests and respiratory pathogen tests, including SARS-CoV-2, were performed on the day of arrival. The polymerase chain reaction test for SARS-CoV-2 was positive. Partial pressure of oxygen (PaO2) was normal on arrival.
Treatment for COVID-19 was initiated, which consisted of hydroxychloroquine (2 x 200 mg), oseltamivir (2 x 75 mg), and azithromycin (1 x 500 mg). On the fourth day of hospitalization, he suffered from tachypnoea (35–40 breaths/min) and hypoxia (85% oxygen saturation with 15 L/min oxygen inhalation). He was admitted to the intensive care unit (ICU), and, while still fully conscious, non-invasive ventilation was employed. Favipiravir (2 x 600 mg) was added to his treatment. On the seventh day of hospitalization, the patient required invasive mechanical ventilation, and he was started on enoxaparin 40 mg twice daily. At the time of aggravation, his D-dimer level was >35.2 mg/L (<0.5 mg/L), ferritin level was 834 ng/mL (22–322 ng/mL) and fibrinogen level was 444 mg/L (150–400 mg/L). Due to progression on lung X-ray, lopinavir/ritonavir (2 x 400/100 mg) was added to his treatment. His worst partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio was 107.
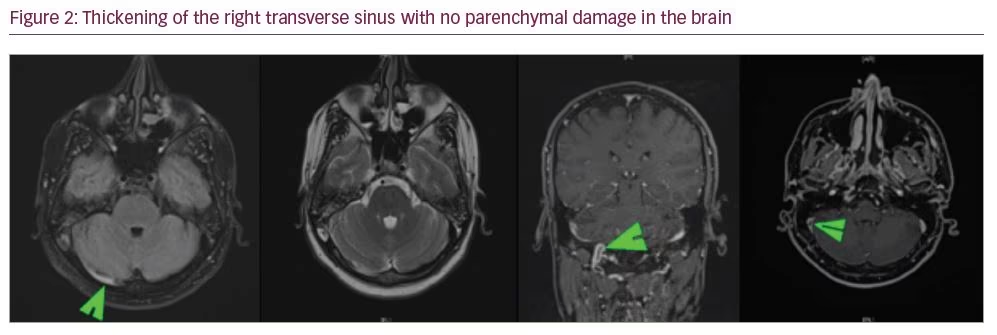
Although sedation was gradually stopped, starting from the 10th day of hospitalization, the patient’s level of consciousness did not improve and resistant high fever remained. On the 11th day of hospitalization, magnetic resonance imaging (MRI) was conducted to exclude any cranial pathology. Cerebral venous thrombosis (CVT) of the right transverse sinus was detected without any infarction or oedema findings (Figure 2). We did not determine any risk factors for CVT, such as a connective tissue disorder or local infection of the head, neck, or sinuses. His antithrombin 3 level was normal (73.7% [70–130%]). Electroencephalogram (EEG) was performed and showed bioelectric disorganization (symmetrical theta waves) in both hemispheres with no epileptic or other specific findings.
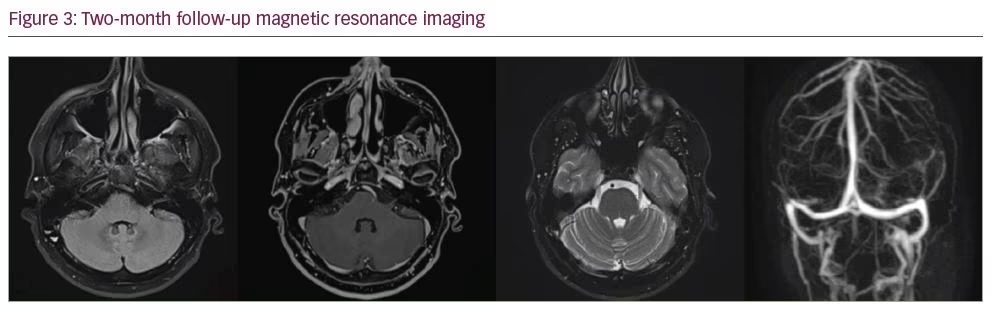
Despite the severe impairment of COVID-19, we could not find any other reason for the long-term delay in the consciousness recovery period. The dosage of enoxaparin was increased from 40 mg twice daily to 80 mg twice daily. On the 23rd day of hospitalization, his consciousness gradually improved, so he was extubated and discharged from the ICU and taken to the hospital ward. On the 26th day of hospitalization, CT angiography of the chest was performed, and there was no sign of pulmonary embolism. He was given symptomatic treatment and intermittent oxygen inhalation, and his neurological condition remained normal until discharge. His 2-month control brain MRI and MR venography showed no abnormality in parenchymal tissue or venous structures (Figure 3).
Discussion
The COVID-19 pandemic has grown rapidly, with various clinical manifestations, including cerebrovascular events and delayed unconsciousness. Neurological complications, including thromboembolic events, have been reported in multiple studies.2,5,7 It is known that delayed unconsciousness is common in severe COVID-19, but in order to exclude any cranial pathology, we performed brain MRI for our patient, and we detected transverse sinus thrombosis. However, the absence of any infarction or oedema led us to believe that this was an incidental finding.
Alterations of consciousness have been reported with severe COVID-19,5,6 but incidence and prevalence studies are not available yet. Neurological consultations are frequently sought for altered consciousness state in patients with severe COVID-19, but neurological examinations, EEG, CT and MRI may not detect the underlying pathology.7 It is possible that in future studies, we will be able to answer how severely COVID-19 affects the brain in some patients. The adverse effects of some sedatives on cognition are well known,8 but the mechanism of prolonged effects is unknown. Some possible factors affecting neural network reintegration may include new drug combinations, longer sedation duration, longer hypoxic state, or impaired drug clearance from liver or kidney.6 Clinicians should consider using EEG guidance for titration of sedatives to avoid delayed unconsciousness. We also need further studies for waking time and prognosis in delayed unconscious state.
We assumed that this patient had no risk factors for CVT, and this condition was secondary to hypercoagulability secondary to COVID-19. A recent study reported that 36.4% of patients with COVID-19 had neurological involvement, such as dizziness, headache, cerebrovascular disease and altered consciousness.2 Cerebrovascular events are mostly detected in older populations because of associated risk factors. CVT is more frequent in young patients with associated risk factors. Unexpectedly, our patient was young and healthy without any known medical or family history for CVT.
It is known that COVID-19 causes thrombotic/thromboembolic events in several ways. The direct and indirect effects of COVID-19 can cause haemostatic abnormalities and disseminated intravascular coagulation, which did not develop in our patient.3,9 The pathology underlying cerebrovascular disease seems multifactorial. Besides the non-specific mechanisms, such as hypoxia, inflammatory response and cytokine secretion, specific viral mechanisms may increase the risk of ischaemic events by infection of vascular endothelial cells and subsequent damage to the vasculature.10 A study showed that inflammatory cytokines, such as interleukin (IL)-2, IL-7 and IL-10, were higher in patients with COVID-19 who were followed in the ICU.1 This inflammation may cause plaque instability.7 This mechanism can explain cerebrovascular events, such as an acute ischaemic stroke, in patients with COVID-19.
In our patient, we did not determine thrombocytopaenia, but the D-dimer level gradually increased up to 35.2 mg/L and C-reactive protein level increased from 15.9 mg/L to 33.8 mg/L. The inflammatory response might be the reason for CVT in our young patient without any neurological risk factors. A retrospective study in 221 patients with COVID-19 reported a few neurological diseases, including acute ischaemic stroke (n=11, 5.0%), cerebral haemorrhage (n=1, 0.5%) and CVT (n=1, 0.5%).11 The demographic features of the young patient with CVT in this study were similar to those in our patient. Although CVT was an incidental finding in our patient, the disease could progress and could cause irreversible results if not detected and properly treated.
To conclude, we must be aware of the possibility of CVT in the context of COVID-19 (especially during the post-viral period) in patients with severe disease course and/or unexplained neurological symptoms in order to initiate proper investigation and treatment. Considering the risk of thrombotic events, neurological manifestations, such as progressive headache, diplopia, papilledema, decreased consciousness and seizure, should promptly raise suspicion of CVT. Further studies are required to confirm a possible causal relationship between CVT and COVID-19 infection. We should also keep in mind that delayed unconsciousness could be seen in patients with severe COVID-19. In necessary conditions, we should minimize sedation and follow-up patients with EEG screening.
Minor post-publication correction: Compliance with ethics statement updated