Epilepsy is one of the most common neurological disorders, affecting around 70 million people worldwide.1,2 Its management is mainly symptomatic, and long-term seizure remission is achieved in most cases.3,4 One-third of patients, however, continue to experience seizures despite adequate treatment.5 Remarkably, the burden of refractory epilepsy has remained stable over the last decades despite increasing therapeutic resources, including third-generation antiseizure medications,6–11 neuro-modulation,12 surgical13 and dietary interventions.14
Cannabis has been used to treat epilepsy since antiquity, and interest in cannabis-based therapies has recently increased. Cannabidiol (CBD) is a main component of the resin of the Cannabis sativa plant. CBD lacks psychoactive effects, carries no risk of abuse liability and has antiseizure properties.15 A plant-derived pharmaceutical formulation of purified CBD oral solution (Epidiolex®, Greenwich Biosciences, Carlsbad, CA, USA) has been recently authorised by the US Food and Drug Administration as treatment, and by the European Medicines Agency (Epidyolex®, GW Pharma, Cambridge, UK) as adjunctive therapy, in conjunction with clobazam (CLB), for seizures associated with Dravet syndrome and Lennox–Gastaut syndrome in patients aged 2 years and older.16,17
Here, we review the pharmacology of CBD and the clinical studies that have assessed its efficacy and safety in patients with Dravet syndrome or Lennox–Gastaut syndrome.
Clinical pharmacology
Pharmacodynamics
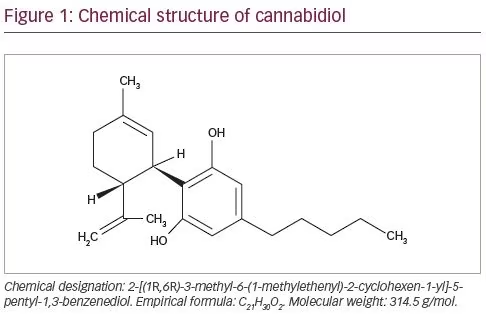
Compared with conventional antiseizure medications, CBD has a different mechanism of action.18 CBD is a 21-carbon terpenophenolic compound that results following decarboxylation from a cannabidiolic acid precursor (Figure 1). CBD has negligible activity at the cannabinoid receptors and exerts its anti-convulsant effects acting on multiple molecular targets. CBD can elicit its effect mainly by the inhibition of T-type calcium channels and the modulation of intracellular calcium ion levels by inhibiting the G-protein coupled receptor 55 and activating the transient receptor potential cation channel subfamily V member 1.17,19,20 CBD can also inhibit the re-uptake and influence the extracellular levels of adenosine, resulting in potential agonist activity at adenosine receptors. Antagonism of α1 adrenergic and m-opioid receptors; inhibition of the uptake of dopamine, norepinephrine, 5-hydroxytryptamine and gamma-aminobutyric acid (GABA); low-potency blockage of sodium channels; and inhibition of endocannabinoid metabolic enzymes and cyclo-oxygenase (COX) 1 and COX-2 are additional activities of CBD, but they are unlikely to represent principal anti-seizure mechanisms of action or occur at supra-pharmacological concentrations.21–24
Pharmacokinetic properties
Following oral administration, CBD has low bioavailability and shows high intra- and inter-individual pharmacokinetic variability. Co-administration of CBD with a high-fat/high-calorie meal can increase maximum serum concentration (Cmax) five-fold, area under the curve (AUC) four-fold, and reduce the total variability compared with a fasted state in healthy volunteers. CBD diffuses into lipophilic tissues and has a high volume of distribution.25 It is highly bound to plasma protein and extensively metabolised in the liver and gut (primarily in the liver) by cytochrome P450 (CYP) isoenzymes, particularly CYP2C19 and CYP3A4, and uridine 5’-diphospho-glucuronosyltransferase (UGT) UGT1A7, UGT1A9 and UGT2B7 isoforms.26 CBD is hydroxylated to the active metabolite 7-hydroxy-CBD (7-OH-CBD), which is further converted to the inactive 7-carboxy-CBD (7-COOH-CBD). A third metabolite, 6-hydroxy-CBD, is found at relatively low levels.27 The major route of excretion is intestinal, and 10–15% of the dose is eliminated in the urine. The main pharmacokinetic properties of CBD are shown in Table 1.
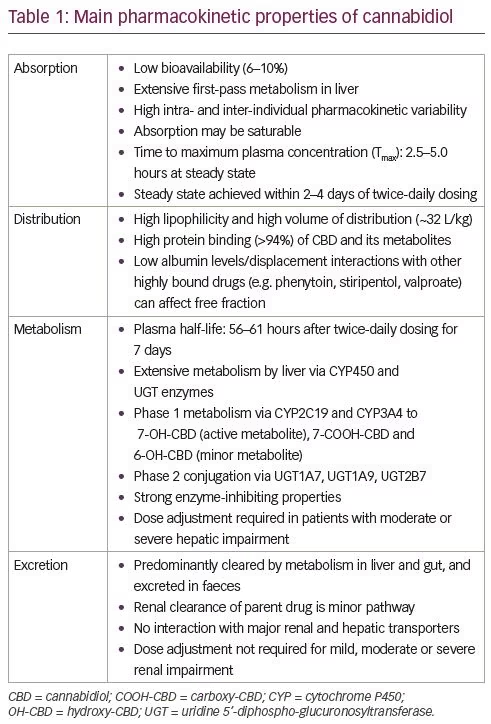
CBD can influence the activity of different enzymes involved in drug metabolism and is a potent CYP inhibitor, especially of CYP1A2, CYP2C9, CYP2D6 and CYP3A4 (Table 2).28 There is a high potential of drug–drug interactions between CBD and both antiseizure and non-antiseizure medications due to the extensive hepatic metabolism and influence of CBD on enzymatic systems.
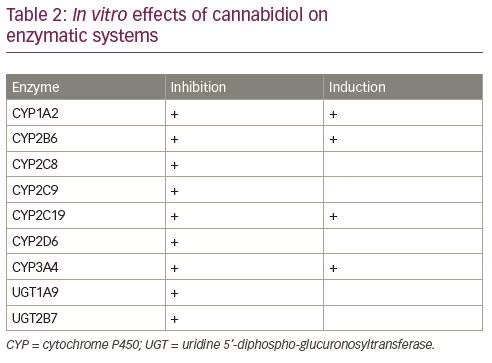
The best characterised pharmacokinetic drug–drug interaction is the bidirectional one between CBD and CLB. CBD can inhibit the CYP2C19 and raise the plasma levels of N-desmethylclobazam (N-CLB), the active metabolite of CLB. CLB leads to an approximately 50% increase in the exposure to 7-OH-CBD, by inhibiting CYP2D6 and glucuronidation.29 The increase in N-CLB exposure is not observed in patients concomitantly treated with stiripentol.30 Stiripentol is a strong CYP2C19 inhibitor and is expected to maximally elevate N-CLB levels. Nonetheless, it is not possible to completely rule out that a further benzodiazepine increase may occur in patients receiving lower than recommended (50 mg/kg/day) doses of stiripentol.31
The concomitant administration of CBD and valproic acid is associated with an increased risk of transaminase elevations. The chemical structure of 7-COOH-CBD reveals similarities with the valproic acid metabolite associated with hepatotoxic properties, and the interaction observed in vitro at the level of hepatic mitochondria could be the mechanism underlying the clinical findings.27
Adjunctive treatment with CBD resulted in increased brivaracetam levels by 95–280% in a case series of five patients, but the clinical relevance of this effect is unknown; only two patients reported mild adverse events (AEs), leading to a reduction of brivaracetam in one patient.32 Concomitant administration of midazolam following repeated dosing of CBD has resulted in a relatively small reduction in the exposure to midazolam and a two-fold increase in the concentrations of 1’-hydroxymidazolam, the active metabolite.33 Linear increases in serum levels of eslicarbazepine, rufinamide, topiramate and zonisamide were observed with increasing CBD dose without any clinical correlates. There were no changes in drug levels of levetiracetam, clonazepam, vigabatrin and pregabalin with CBD dose titration.34 Although drug–drug interactions may potentially occur with ethosuximide, lacosamide, lamotrigine, perampanel, phenobarbital and phenytoin, no significant changes have been found with CBD dose titration.34 A summary of the main known interactions between CBD and antiseizure medications is provided in Table 3.
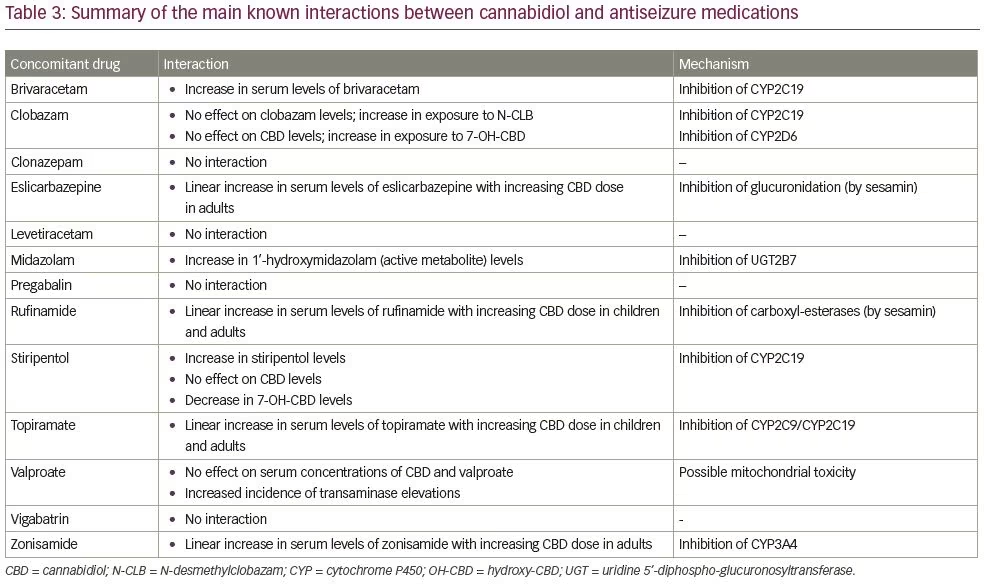
Interactions between CBD and non-antiseizure drugs can be hypothesised based on metabolic pathways and actions on enzymatic systems. Although dedicated drug–drug interaction studies have not been conducted, the co-administration with moderate or strong inhibitors/inducers of CYP3A4 and CYP2C19 is expected to increase/reduce CBD plasma concentrations. Reduction/increase in CBD dosages should be considered based on tolerability and efficacy.
Clinical evidence of efficacy and safety
Randomised controlled trials
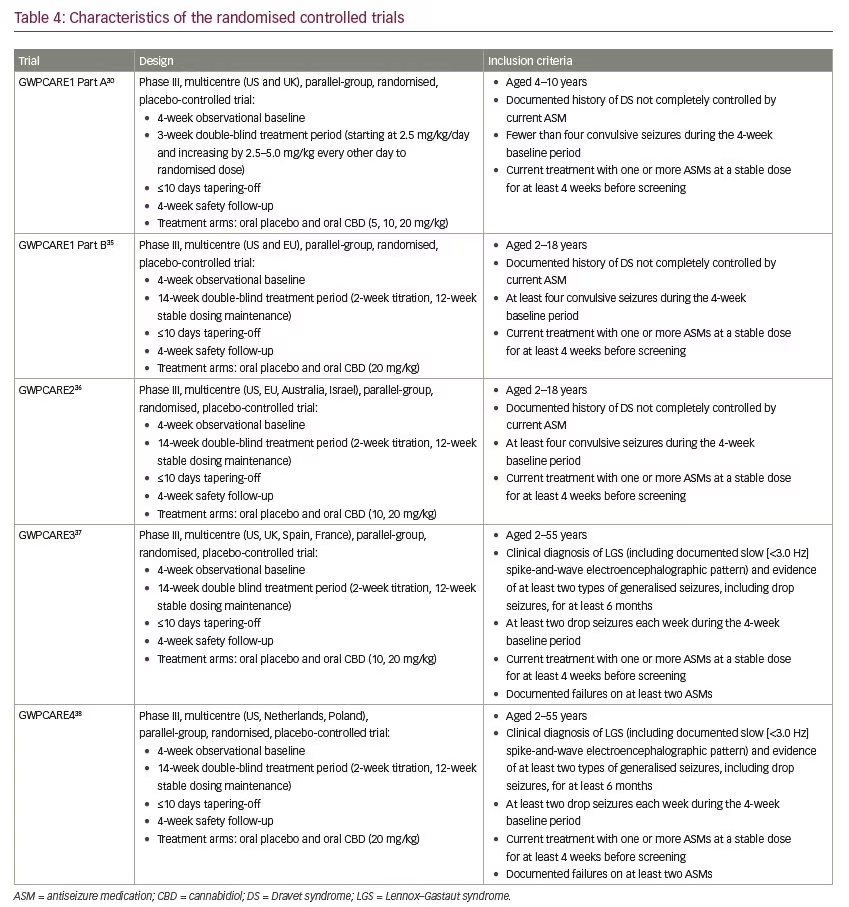
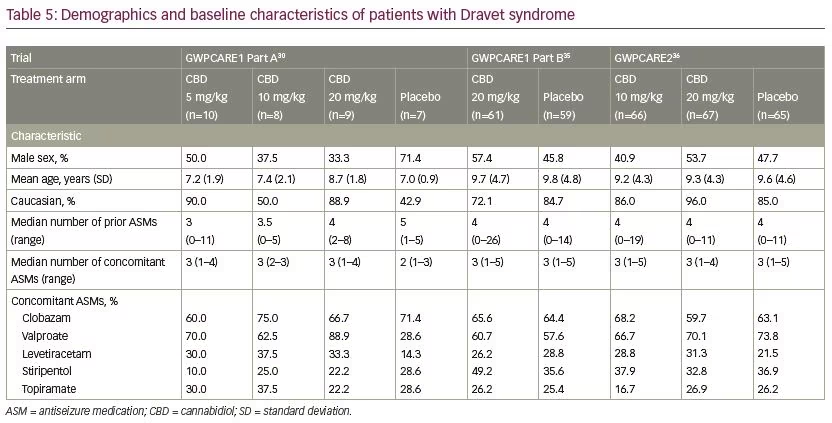
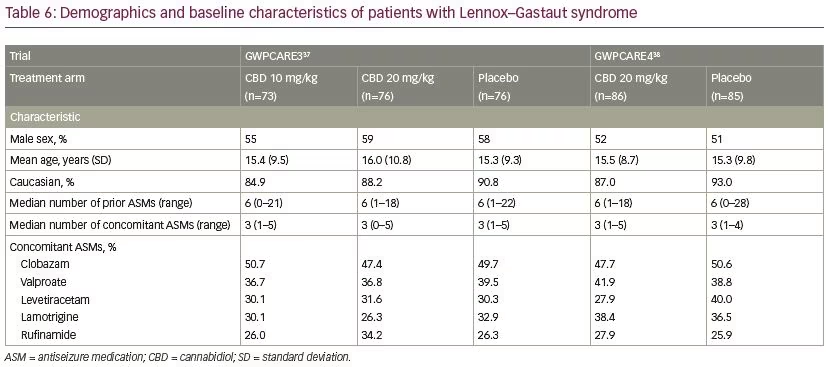
Five multicentre, randomised, double-blind, placebo-controlled trials evaluated the efficacy and/or safety of adjunctive CBD in patients with Dravet syndrome30,35,36 and Lennox–Gastaut syndrome.37,38 In all trials, CBD oral solution (100 mg/mL) was added to the antiseizure treatment. Characteristics of the trials and patients are summarised in Tables 4–6.
Antiseizure efficacy
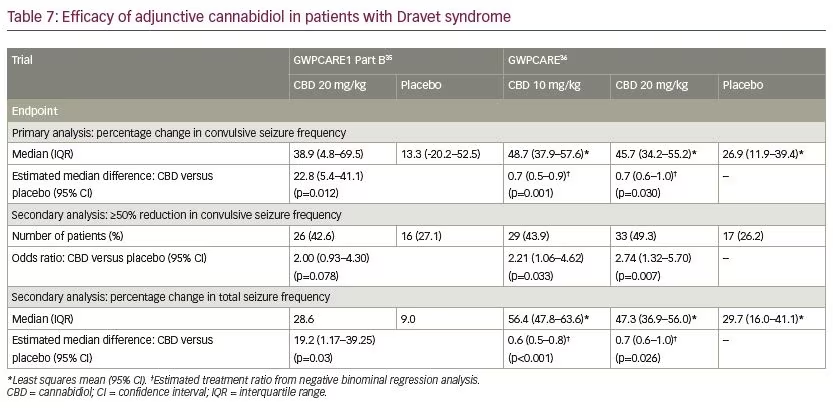
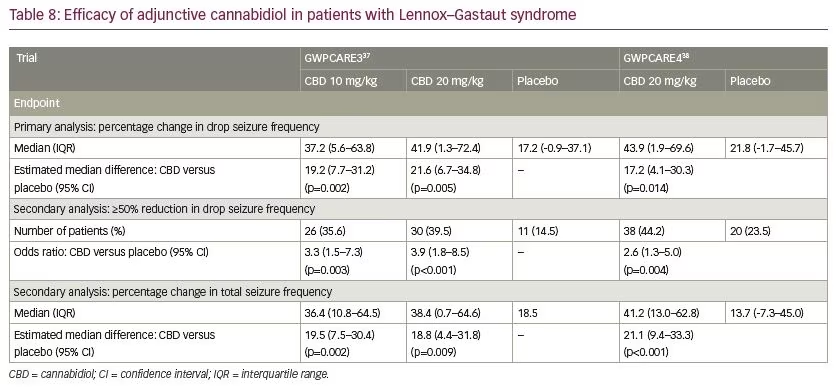
In patients with Dravet syndrome, adjunctive CBD was associated with a greater reduction in convulsive and total seizure frequency (Table 7). Across the trials, the percentage of participants who presented a ≥50% decrease in convulsive seizure frequency was 45.4% with adjunctive CBD and 26.6% with placebo (risk ratio [RR] 1.69, 95% confidence interval [CI] 1.21–2.36; p=0.002).39 In the Lennox–Gastaut syndrome cohort, adjunctive CBD resulted in a significantly greater decrease in drop and total seizure frequency than placebo (Table 8). The overall percentage of participants who achieved ≥50% decrease in drop seizure frequency across all the trials was 40.0% with CBD and 19.3% with placebo (RR 2.12, 95% CI 1.48–3.03; p<0.001).40 Notably, CBD treatment improved the baseline status of patients with Dravet syndrome or Lennox–Gastaut syndrome as assessed by the Patient or Caregiver Global Impression of Change questionnaire.41
One major issue in the clinical use of CBD is the concomitant administration of CLB, which is often a component of the regimen used to treat Dravet syndrome and Lennox–Gastaut syndrome.42,43 Increased systemic levels of N-CLB and 7-OH-CBD might enhance the pharmacological effects. CBD is also a positive allosteric modulator of GABAA receptors and a pharmacodynamic interaction occurs, where co-administration of CBD further enhances GABAA-mediated inhibitory activity.44 Significant drug–drug interactions between CBD and CLB have overall raised doubts about the intrinsic antiseizure activity of CBD and imposed prescription limits in Europe.17,45 In this regard, a recent systematic review and meta-analysis showed that adjunctive CBD was associated with a higher rate of seizure response in comparison to placebo at the dosage of both 10 and 20 mg per kg/day in patients with Dravet syndrome or Lennox–Gastaut syndrome, irrespective of the concomitant use of CLB.46
Safety and tolerability
During pivotal studies, drug discontinuation and AEs were more common in the CBD than placebo groups, mostly at the highest dose. Most AEs were mild or moderate, and usually resolved with treatment. The most frequent AEs associated with CBD were somnolence, decreased appetite and diarrhoea (Table 9).41 Somnolence was more common among participants who were also taking CLB and a decrease in the dose of the benzodiazepine was more frequently observed among patients treated with CBD than placebo. Drug–drug interactions leading to an increased serum-level of CLB and its metabolites are likely to contribute to the development of somnolence or sedation. As it is clinically difficult to distinguish benzodiazepine toxicity from CBD-related AEs, therapeutic drug monitoring is recommended before the administration of CBD and after any dosage increase.
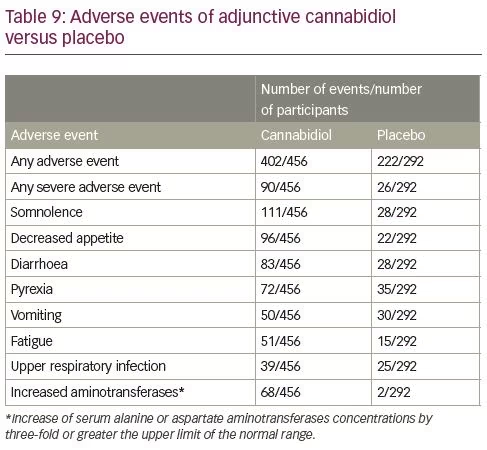
CBD has been associated with raised liver enzymes, which was documented to be the most common severe AE and explained half of the treatment withdrawals in the pivotal studies.41 Transaminase elevations generally appear during the first 1–3 months of treatment, and rare cases have been known to develop up to 200 days after treatment.47 Resolution of transaminase elevations usually occurs after CBD is tapered or discontinued, or the dose of a concomitant antiseizure medication is reduced; in about one-third of the cases, transaminase elevations have resolved spontaneously during continued treatment with CBD, without dose reduction.47
Risk factors for transaminase elevation include concomitant use of valproic acid and CLB, CBD dose, and baseline transaminase levels. Most cases of aminotransferases elevations developed in participants taking concomitant valproic acid, and some resolved during CBD treatment after the reduction in valproic acid dosage.48 Concomitant use of CLB also increases the risk of transaminase elevations, albeit to a lesser extent. Transaminase elevations greater than three times the upper normal limit occurred in 30% of the participants taking both concomitant valproic acid and CLB, about 20% of the participants under concomitant treatment with valproic acid (without CLB), 4% of the participants concomitantly administered with CLB (without valproic acid), and 3% of the participants treated neither with valproic acid nor CLB. Transaminase elevations are dose-related: they have been known to occur in 15–20% of participants treated with CBD at the daily dose of 20 mg/kg and 1% of participants treated with CBD at the daily dosage of 10 mg/kg.48 Patients with levels of liver enzymes above normal limits before starting treatment have also been found to have higher rates of further elevations: the incidence of treatment-emergent elevations of transaminases in patients taking CBD (20 mg/kg/day) was 30% and 10% when baseline transaminases levels were above and within the normal range, respectively.48
Open-label studies
Patients with Dravet syndrome or Lennox–Gastaut syndrome who completed the randomised controlled trials were invited to enrol in a long-term, open-label extension trial (GWPCARE5; ClinicalTrials.gov identifier, NCT02224573). Based on response and tolerance, CBD could be reduced or increased up to 30 mg/kg/day. Clinically meaningful reductions in seizure frequency were maintained on long-term treatment up to 48 weeks according to pre-specified interim analyses, and safety profile was similar to the one observed in pivotal trials.49,50
Expanded access programme results provide further supporting evidence regarding the long-term effectiveness of add-on CBD in subjects diagnosed with Dravet syndrome or Lennox–Gastaut syndrome.51 Over a median treatment duration of 78.3 weeks (range 4.1–146.4 weeks), treatment withdrawal occurred in 28% of the patients, primarily due to lack of efficacy. Between weeks 12 and 96, median daily CBD dose ranged from 21–25 mg/kg. At 12 weeks, add-on CBD reduced median monthly major motor seizures by 50% and total seizures by 44%, with consistent reductions through 96 weeks. The proportions of patients with ≥50% and 100% reductions in major motor seizures were 53% and 6% at 12 weeks, and the proportions with corresponding reductions in total seizures were 46% and 5%. The safety profile was acceptable, and somnolence and diarrhoea were the most frequent AEs.51
Different studies have evaluated changes in seizure frequency and occurrence of AEs associated with highly purified CBD as an add-on treatment in patients with treatment-resistant epilepsy.52–60 Marked heterogeneity was seen in treatment duration and drug daily doses, ranging up to 50 mg/kg, and the prevalence of patients with Dravet syndrome or Lennox–Gastaut syndrome was not always provided. Overall, CBD improved seizure control and was effective in reducing seizure frequency with or without concomitant CLB.61,62 The most common AEs observed across the cohorts included somnolence, decreased appetite and gastrointestinal symptoms; no major safety concerns emerged. Beneficial effects on sleep, behaviour, motor skills, social interactions, mood and quality of life were observed and were independent of treatment response. Long-term administration of pharmaceutical-grade CBD was cognitively well-tolerated; modulation of the activity and functional connectivity related to attentional control processes was also suggested to improve cognitive performance.63,64 Even with limitations related to uncontrolled nature and non-randomised design, open-label studies can reflect and provide useful information about the effectiveness of CBD in real-world clinical practice.65
Dosage, administration and use in specific populations
CBD is administered at the starting dosage of 2.5 mg/kg (twice daily; daily dosage of 5 mg/kg). After 1 week, the dose can be increased to a maintenance dose of 5 mg/kg (twice daily). Patients who tolerate CBD at 10 mg/kg/day and require further reduction of seizures may benefit from a dosage increase up to a maximum recommended maintenance dosage of 10 mg/kg twice daily, in weekly increments of 2.5 mg/kg twice daily, as tolerated.48 Due to the risk of hepatocellular injury, serum transaminases and total bilirubin levels should be assessed in all patients before initiating CBD. Liver enzymes should be monitored at 30, 90, and 180 days from starting CBD, and periodically thereafter or as clinically indicated. More frequent monitoring can be indicated in patients who are taking valproic acid or have elevated liver enzymes at baseline.48
Randomised controlled studies of CBD in the treatment of Dravet syndrome and Lennox–Gastaut syndrome did not enrol participants with aged <2 years or >55 years. According to a general rule, dose selection in elderly patients should be cautious, usually starting at the low end of the dosing range, considering the greater frequency of decreased hepatic, renal, or cardiac function, comorbidities and concomitant drug therapies.66–68
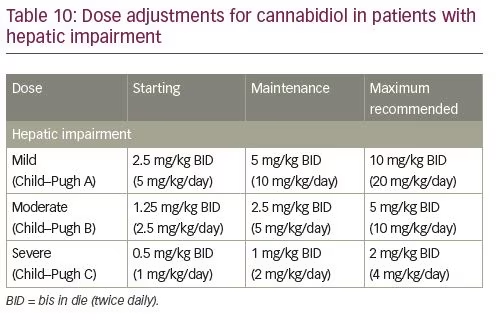
In patients with moderate or severe hepatic impairment, CBD dose adjustment is recommended due to the increase in exposure and slower titration may be necessary; no adjustment is required in patients with mild hepatic impairment (Table 10).48 The labelling for CBD oral solution currently does not list any black box warnings. CBD is contraindicated in patients with a history of hypersensitivity to CBD or any other ingredients in CBD oral solution.
Conclusion
The authorisation of CBD for the treatment of two forms of childhood epilepsy is a turning point in the use of cannabinoids to manage epileptic conditions. CBD oral solution (Epidiolex) is the only pharmaceutical substance deriving from the cannabis plant that has undergone review through the approval processes and represents the first in a new class of antiseizure medication. Remarkably, results cannot be extrapolated to other cannabis-derived products and non-purified forms of medical marijuana or its components. Indeed, the evidence about the efficacy and safety of cannabis-based products or synthetic cannabinoids for medicinal use like CBD-enriched cannabis oil containing CBD plus tetrahydrocannabinol (THC), TIL-TC150, and dronabinol, and non-medicinal cannabis or CBD products is mainly limited to uncontrolled studies, small series, case reports and surveys, and does not allow one to draw firm conclusions.65,69,70 In many cases, unspecified products with unknown composition and dosage, including oral cannabis extracts and artisanal or hemp-based CBD preparations, were used. In particular, non-medicinal CBD products are widely sold as herbal remedies but are not scheduled and regulated as medicinal products. They are widely available on the internet and from health food retailers, but they lack quality standards; they contain CBD and THC in varying quantities and proportions, often at concentrations wildly different from those reported in their labels,71 and the amount of CBD is typically far lower than in clinical trials.15 The use of such products can be also associated with the occurrence of AEs and intoxication from psychoactive constituents, including seizure exacerbation.72
Recently, CBD has also been demonstrated to significantly reduce seizures associated with tuberous sclerosis complex,73 and research is ongoing to evaluate this drug in infantile spasms. Additional research in different types of epilepsy, including refractory focal epilepsy, is needed to further explore and understand the potentialities of CBD.