it is from this group doubtless that the generally unfavorable impression regarding gliomas as a whole has been gained. It is not only the largest single group in the series … but at the same time is one of the most malignant … In the five unoperated cases, the average duration of life from the onset of symptoms was only three months, which speaks well on the whole for the average survival period of twelve months for those surgically treated.”2
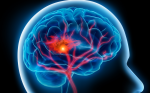
Since their seminal work, the median survival of 12 months has not changed markedly. Both data from the 1960s 3 and current data4 confirm that the extent of surgical resection is an important prognostic factor. However, as Bailey and Cushing observed, GBMs have “infiltrating propensities, and … when enucleation is attempted, the growth is found at the depth to spread into and merge with the normal cerebral tissue without recognizable demarcation.”2 In prior eras, radical surgical excisions, including removal of the entire cerebral hemisphere containing the tumor,5 were occasionally attempted, yet patients who survived the hemispherectomy died of recurrent tumor,6 clinically proving the importance of the histologic observation that tumor cells invade throughout the brain. In the modern age, brain imaging may disclose macroscopic tumor in the opposite hemisphere (see Figure 1) or even gliomatosis cerebri – literally a brain full of tumor. In the years leading to up to World War II, the German pathologist Scherer, whose scientific discoveries were tainted by his Nazi activities,7 described ‘secondary structures’8,9 that further characterized invasive tumor cells. These structures are ‘secondary’ because they are dependent for their formation on underlying normal brain structures, as opposed to ‘primary’ structures of the tumor such as pseudopalisading necrosis and microvascular proliferation. Examples include perineuronal and perivascular satellitosis (accumulation of tumor cells around neurons and blood vessels), subpial spread, and intrafascicular tracking such as infiltration along corpus callosum and other white matter tracks (see Figure 2).
The gliomas are assigned a grade by the World Health Organization (WHO) depending on histologic features that predict behavior.This classification scheme is derived from the clinicopathologic studies of Bailey and Cushing.2 Grade I tumors, such as juvenile pilocytic astrocytomas, are generally focal rather than diffuse and are potentially curable by surgical excision.WHO grade II–IV tumors are diffusely infiltrative.WHO grade III–IV tumors are termed ‘high grade’ or malignant. GBMs, or grade IV astrocytomas, are the most aggressive subtype.
Advances in surgical technique, imaging, and targeting of radiotherapy are important contributions to local control. However, changing GBM from a disease that kills quickly to one that can be managed as a chronic illness like hypertension or diabetes mellitus will require systemic therapies targeting tumor cells infiltrating throughout the brain, such as chemotherapy, immunotherapy, and small molecule pathway inhibitors.
Present
Currently, treatment for GBM involves both local and systemic therapy. Surgery and partial brain radiotherapy are the standard locally directed therapies. Some physicians also advise intra-operative placement of chemotherapy containing polymers (i.e. Gliadel ‘wafers’) directly into the surgical bed in an attempt to prolong local control.10 While there is a modest survival benefit, the use of these polymers remains controversial because of the potential for toxicity. Other treatment modalities that target disease localized to the surgical bed or the surrounding area have included brachytherapy and stereotactic radiosurgery (with either a linear accelerator or gamma-knife), neither of which are commonly advised. Convection-based chemotherapies delivered by catheter infusion, such as local delivery of pseudomonas exotoxin linked to either interleukin 13 (IL-13) or transforming growth factor alpha (TGFa),11 are available in clinical trials for some patients.These trials take advantage of differences in the expression of proteins (such as growth factor receptors) on the surface of residual tumor cells in the periphery of the operative bed to deliver the toxin to tumor cells but spare normal brain.
Contrast enhanced magnetic resonance image (MRI) of the brain demonstrating a large glioblastoma multiforme (GBM) with a smaller site in the contralateral hemisphere. This infiltrative nature of GBMs, essentially effecting the entire brain, underscores the failure of even radical surgery, such as hemispherectomy, to effect cure.
By contrast, systemic chemotherapy targets tumor cells beyond the reach of local therapies. The most commonly prescribed systemic chemotherapy for GBM is temozolomide (Temodar®), an alkylator that became available during the last decade. The effectiveness of temozolomide in the management of GBM at diagnosis was recently demonstrated by a large multinational study.4 A modest survival benefit of 2.5 months for concurrent temozolomide with radiotherapy (14.6 months median survival) was observed relative to radiotherapy alone (12.1 months median survival).4 In addition, while the survival benefit was still present two years after diagnosis, only 10.7% of patients were progression-free and only 26.5% of patients were alive at that point.4 While systemic chemotherapy improves the outcome for some patients, long-term disease control therefore remains elusive.
Discoveries during the last several years have improved the understanding of glioma and general cancer biology markedly. Generally, a cancer comprises cells that either divide or survive when they should instead undergo either cell cycle arrest or die. These abnormalities are also not mutually exclusive, and most cancers, including GBMs, are driven by several molecular abnormalities. The signal to divide is typically provided by a growth factor (ligand). Examples include TGFa, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF). Such ligands interact with cells through receptors including EGF receptors (EGFRs), PDGF receptors (PDGFRs), and VEGF receptors (VEGFRs). Receptor activity is linked with cellular processes such as mitosis or invasion by signal transduction cascades. Examples of signal transduction cascades important in human GBMs include those activated by the oncogenes RAS, AKT, and SRC.12 In cancer cells, these pathways are disrupted through several mechanisms. For example, EGFR is overexpressed in up to 92% of astrocytomas,13 and up to 62% of GBMs express EGFRvIII,14 a mutant receptor that is active independently of ligand. Coexpression of EGF and EGFR15,16 in GBMs leads to a potential autocrine loop.An analogous loop is created by PDGF and PDGFR co-expression in up to 94% of high-grade oligodendrogliomas.17–19 Regardless of ligand or receptor status, close to 100% of GBMs exhibit activation of RAS,20,21 and approximately 70% exhibit activated AKT,21,22 the latter typically through loss of the tumor suppressor gene phosphatase tensin homolog on chromosome ten (PTEN),23–25 which normally represses AKT activation. SRC is detected in 67% of GBMs.26 Finally, control over cell division is normally maintained by tumor suppressors such as inhibitor of CDK4A (INK4A) and its alternative reading frame (ARF), as well as p53, which also contributes to DNA repair and apoptosis, and other enzymes. Disruptions of normal cell cycle control of one form or another have been observed in almost all GBMs.12,27 Moreover, modeling of gliomas in mice has demonstrated that abnormalities of ligands, receptors, signal transducers, and proliferation cause gliomas. For example, combined activation of RAS with AKT in glial progenitors is sufficient to induce GBMs in mice,22 and transgenic expression of activated forms of RAS28 or SRC29 in glia leads to GBMs following spontaneous development of cooperative oncogenic abnormalities. Modeling has also demonstrated that PTEN loss is functionally equivalent to AKT activation 30 when combined with activated RAS. PDGF overexpression in glia causes high-grade oligodendrogliomas 31,32 that also exhibit pathologic features of GBMs including pseudopalisading necrosis and microvascular proliferation. The threshold to tumor formation is lowered by disruption of Ink4a-Arf or p53 expression.31,33,34
As more is learned about glioma biology, small molecule inhibitors are being developed that target the causal pathways.35 For example, several inhibitors of extracellular growth factor receptors are under investigation in clinical trials.These include the EGFR inhibitors erlotinib (OSI- 774/Tarceva), gefitinib (ZD-1839/Iressa), and lapatinib (GW572016). The PDGFR inhibitors imatinib (STI- 571/Gleevec) and PTK787, both of which have other targets, are also in use. Signal transduction cascade blockers are also being studied. One example is R11577, which targets the enzyme that activates RAS.Rapamycin (sirolimus), CCI-779 (temsirolimus), and Rad-001 (everolimus) target mTOR, one of the key enzymes activated by AKT.
Unfortunately, despite initial enthusiasm, treatment of GBMs as well as systemic malignancies with these small molecule inhibitors as single agents has generally been disappointing. For example, published interim and final reports of trials involving gefitinib,36,37 erlotinib,38–40 imatinib,41,42 PTK787,43,44 and CCI-77945 monotherapy for recurrent high-grade gliomas have not shown response or survival rates that are markedly superior to those observed with traditional chemotherapies such as temozolomide4,46–48 or carmustine (BCNU).49 Yet there are individual patients treated with these agents who experience durable objective responses or sustained stable disease.Therefore, these agents are likely to have a role in GBM management.
Future
In addition to surgical resection and radiotherapy, the future of GBM therapy is likely to involve both additional measures to improve local control (such as convection or catheter delivery of antitumor agents into the operative cavity) and systemic treatment to address infiltrative disease distant from the main tumor bed. However, a major thrust of research will be tissue analyses looking for molecular features that predicts sensitivity of GBMs to either traditional chemotherapies or small molecule inhibitors. Tailoring therapy with specific drugs to those patients is most likely to improve response rates and spare patients who are unlikely to benefit the expense and potential toxicity of these agents. Determination of a molecularly effective dose (MED, inhibits a pathway), may also be more useful than the traditionally used maximally tolerated dose (MTD).
An example of a molecular prognostic factor is loss of heterozygosity for chromosomes 1p and 19q in anaplastic oligodendrogliomas, which predicts both sensitivity to chemotherapy and radiation as well as longer overall survival.50 Consequently, some neurooncologists are currently using results of 1p/19q analysis to guide therapy,51 although this remains an area of controversy. Other genomic alterations are also predictive – PTEN loss is associated with poor survival for patients with anaplastic oligodendrogliomas52 and likely to predict poor outcome from GBM.23,53,54 More recently it was reported that GBMs, in which O6- methylguanine-DNA methyltransferase (MGMT) expression was silenced by gene methylation, were more sensitive to temozolomide than tumors with unmethylated MGMT. The likely explanation is that MGMT may counteract temozolomide activity by removing alkyl groups on DNA.55 It is unclear whether MGMT methylation impacts sensitivity of other glioma subtypes to temozolomide, yet MGMT methylation status may be used in the near future to guide therapy.
(A) Pseudopalisading (arrow) necrosis (arrow head) and (B) microvascular proliferation (arrow) are the classic histologic findings in glioblastoma multiforme (GBM). Secondary Scherer structures (C) involve tumor cells (arrow heads) accumulating around blood vessels (BV, long arrow) and neurons (N, long arrow) in a low grade (WHO grade II) oligodendroglioma. Such perivascular and perineuronal satellitosis, along with intrafascicular growth and subpial accumulation (not shown), contribute to the diffusely infiltrative nature of gliomas throughout normal brain structures. Photomicrographs were kindly provided by Mark A Edgar, MD, Department of Pathology, Memorial Sloan-Kettering Cancer Center.
Individualized medicine determined by molecular rather than simply histologic phenotype may also guide therapy with small molecule inhibitors. Somatic mutations in exons 18-21 of EGFR are associated with sensitivity of lung cancer to gefitinib 56–58 or erlotinib.58 However, the authors and others have not found these mutations in gliomas.34,57,59,60 Efforts are under way to identify the molecular features that predict sensitivity of GBMs to EGFR and other receptor tyrosine kinase (RTK) inhibitors.
An extracellular ligand such as EGF,TGFa, or PDGF induces dimerization of receptors such as EGFR or PDGFR. Receptor stimulation activates intrinsic tyrosine kinase (TK) activity and EGFR and PDGFR are therefore called receptor tyrosine kinases (RTKs). RTKs then activate the AKT, SRC, and RAS signal transduction cascades.Tumor cell growth is driven by ligand or receptor overexpression, constitutively activating receptor mutations (e.g. EGFRvIII), or signal transduction activity. Pointed (green) and block (red) arrows indicate pathway activation and inhibition, respectively.The inhibitors shown and others are under investigation in the treatment of GBMs.
Response and survival rates may also be improved through combination therapy. For example, preliminary data suggests that concurrent therapy with imatinib (PDGFR/VEGFR inhibitor) and hydroxyurea (a more traditional chemotherapy) is more effective than imatinib monotherapy. A small series with 14 evaluable patients with recurrent GBMs demonstrated a disease control rate (complete response or partial response or stable disease) of 64% for patients treated with this combination.61 By contrast, imatinib monotherapy led to a disease control rate of 29%.42 Larger trials of this and other combinations, such as temozolomide with PTK787, are underway.
Conclusions
Bailey and Cushing observed that gliomas become more aggressive with time, writing “all of these lesions, so far as our records permit us to judge, show an increasing degree of malignancy, the recurrent tumors giving evidence of more active cell division than the original lesion.”2 It is now understood that the accumulation of molecular abnormalities1 underlies the increasingly aggressive clinical behavior of an individual patient’s glioma over time. Moreover, while GBMs may be histologically identical, they are molecularly distinct,51 and each tumor may require individually tailored treatment with the combination of agents predicted to impact multiple molecular abnormalities. Successful therapy of a molecularly complex disease such as GBM may also require simultaneous administration of multiple agents, including both traditional chemotherapies and several pathway inhibitors.”