Recently available biological therapies mandate further refinements in MRI images. Basic to tumor growth in the brain is the recruitment of new vessels, which investigators have attempted to prevent with a plethora of angiogenesis inhibitors provided in phase I/II clinical trials.1 Similarly, conventional therapies like radiotherapy are at least partly mediated by microvascular damage.2 Of newer MRI modalities, perfusion MRI has emerged as a unique marker of tumor-induced blood vessels and their function. MRIbased perfusion measures the vascularity within a tumor, as well as its component heterogeneous parts. In this review, we will present the principles of perfusion MRI, its application in brain tumor imaging, and the areas of translational application.
Perfusion MRI
At initiation, tumors in a pre-vascular phase are supplied by oxygen and nutrients that diffuse from pre-existing normal vessels.When the tumor reaches a critical size of approximately 1-4mm diameter,3,4 the resultant ischemia leads to the secretion of angiogenic factors, which in turn leads to proliferation of new vessels. These factors, such as vascular endothelial growth factor (VEGF), recruit and maintain tumor vessels, which are characterized by a tortuous and branched structure.5,6 Both in vitro and in vivo, ‘new’ vessels (neovasculature) exhibit increased blood volume and permeability compared with normal vessels.
Vascular imaging provides a visual correlate of vessel dilatation, increased blood vessel volume, and permeability. To evaluate these changes as well as the effects of vessel-specific therapies, neuroradiologists provide research-based measures of cerebral blood volume (CBV), cerebral blood flow (CBF), the mean transit time of contrast (MTT), and transfer constants of contrast agents leaving and re-entering these blood vessels (k1, k2). Of these parameters, blood volume and permeability are commonly applied in patient studies. Blood volume measures the aggregate size of the vascular space within designated areas, while the permeability function informs about the integrity of vessels and their ‘leakiness’ to contrast agents. Each function is derived from the analysis of signal changes over time of contrast agents that are injected into the vein and subsequently reach the area of interest. If no contrast flows to the imaged areas or the molecular size of the contrast agent is extremely large, permeability estimates cannot be made. In general, measurements of vascular images assume two compartments of contrast flow:(k1) describes contrast that initially moves out of the vessel into the extravascular space and (k2) the contrast that moves from the extravascular space back into the vessel.7 The rate of change of contrast in the extravascular space is described by the following formula:
Rate of change of contrast = k1 x Cb – k2 x Ae
where Cb = contrast concentration in blood and Ae = amount of contrast in the extravascular space.
In clinical settings, two different contrast-based MRI techniques are used: T2*-weighted dynamic susceptibility MRI and T1-weighted dynamic contrastenhanced perfusion imaging. Both measure the pharmacokinetics of contrast that passes through a predefined volume. In general, T2*-weighted images,obtained during ultrafast scans, reflect contrast in the vascular bed, while T1-weighted dynamic contrast images measure the intravascular contrast and the resulting leakage from the vessels.T2*-weighted dynamic susceptibility MRI requires rapid serial imaging and high temporal resolution.This technique usually employs a very fast imaging technique called echo-planar imaging. Following rapid injection (3–7ml/second) of 0.1–0.3mmol/kg gadolinium, images are obtained every 1–2 seconds to produce maps of relative CBV, CBF, and MTT using an arterial input function and a deconvolution algorithm. The quantification of the arterial input function, as the contrast passes through the imaged tissue section, is sensitive to changes in the circulation and recirculation of contrast.As contrast courses through the vascular tree and leaks into the extravascular space, the signal decreases and the relative concentration of contrast in the vessels and extravascular tissue can be monitored over time. Contralateral normal white matter is used as a reference. By integrating the change in signal over time for each voxel, a CBV map is produced.This provides 10–12 cross-sectional images through the tumor.8 However, this approach is limited in certain high-grade tumors with blood–brain barrier leakiness, because the theoretical model is based on the assumption that the contrast agent remains within the intravascular space. Rapid leaking of large amounts of contrast into the extravascular space shortens the T1 effect and increases the signal baseline.What emerges is a falsely low relative CBV (rCBV) estimate.8 This leakiness can be corrected by mathematical models or by shifting the baseline by pre-injection of a small amount of gadolinium (0.05mmol/kg) to reduce the T1 shortening effects. Previously, the agent sprodiamide was used to increase T2* effects. Newer paramagnetic contrast agents with varying size and barrier constraints may complement mathematical correction models.9
T1-weighted dynamic contrast-enhanced perfusion imaging relies on the enhancement of tissue as a function of the volume of blood vessels and their permeability. A low dose of gadolinium (0.05–0.1mmol/kg) is given slowly (2ml/second) and gradient echo T1-weighted sequences are used to obtain images every 8–30 seconds.10 The study time for T1-weighted dynamic perfusion imaging is long, as late scans reflect the equilibrium between contrast concentration in the vessels and interstitium as well as the movement of contrast back into vessels (k2). The resulting maps provide images of vascular volume,CBV, the capillary transfer constants k1 and k2, and the space in which contrast is distributed. This approach is not confounded by high vessel permeability and leakiness, since leakage into the interstitium is quantified by late scans. Therefore, mathematical models or pre-injection of contrast are not needed to correct for vascular leakage.However, recent improvements in the temporal resolution of T1-weighted scanning may advance T1- weighted perfusion imaging to the point where it will replace T2*-weighted imaging. Further advantages and disadvantages of T2* and T1-based imaging techniques are listed in Panel 1.
Arterial spin labeling (ASL), a third method of perfusion imaging, is entirely independent of the use of contrast agents. Red blood cells, rather than injected contrast, are used as an endogenous label. Blood, including its component water and red blood cells, is labeled in the neck or below the site of tumor using an inversion recovery pulse.11,12 An extra MRI coil is used to continuously label the blood or pulse labeling is performed without an extra coil. The advantages and disadvantages of pulsed versus continuous labeling methods are listed in Panel 2.
The images include both the inflow of the labeled blood into the tissue of interest and the measurement of spins on a tissue level. Thus, ASL does not rely on tumor vessel permeability, nor requires correction for leakage, is independent of contrast agents, and offers the potential of characterizing regions of vascular leak with an accuracy at least that of rCBV maps obtained from T2*-weighted MRI. On the other hand, the technique requires imaging times of 4–8 minutes per slice, offers low spatial resolution compared with T2* rCBV maps, a low signal-to-noise ratio, and requires specialized hardware.13 A number of animal models of brain tumors have begun the validation of measurement of vascularity, blood volume, and vascular permeability. Various methods, including intravital microscopy and direct visualization of blood vessels,5 doppler ultrasound,14 and perfusion MRI,15 have been explored. For example, the following therapies have been validated: athymic rats bearing orthotopic U87MG gliomas treated with anti-VEGF antibodies demonstrated slowed tumor growth.T2-weighted volumetric studies and dynamic contrast-enhanced T1-weighted imaging revealed reductions of vascularity, fractional blood volume, and vascular permeability.16 Similarly, dynamic contrast-enhanced MRI (DCE-MRI) results have been shown to correlate well with microvessel histologic density,17 and to have a high level of reproducibility in animal models and humans.17,18
CBF = cerebral blood flow, CBV = cerebral blood volume, nCBV = normalized CBV, rCBV = relative CBV, DCE-MRI = dynamic contrast-enhanced magnetic resonance imaging, SE = spin echo, GE = gradient echo, EPI = echo-planar imaging, DSC-MRI = dynamic susceptibility contrast-enhanced MRI; rR = relative recirculation, SPGR = spoiled gradientrecalled acquisition, FSE = fast-spin echo.
While some studies in humans have evaluated the response to antiangiogenic therapies with conventional imaging modalities only,19,20 recent trials have also looked at vascular parameters as potential biomarkers for treatment response as assessed by perfusion MRI. For example, DEC-MRI was used to determine contrast kinetics in previously untreated inflammatory breast cancer. Administration of the humanized anti-VEGF antibody, bevacizumab, resulted in reduction of permeability and extravascular volume.21 In systemic renal cell carcinoma, the antiangiogenic potential of interleukin (IL)-2 and IL-12 therapy has been evaluated with DECMRI as early as within the first two weeks of therapy (Choyke P et al., personal communication). Similarly, tumor recurrences may be heralded by the appearance of focal areas of elevated CBV. Other studies have confirmed the strong correlation between decreased permeability on rCBV maps and tumor size in response to therapy with DEC-MRI and T2* techniques.22–24 Thus, it has been suggested to include DEC-MRI as a surrogate biomarker for clinical outcome in the development of antiangiogenic therapies.
New studies have also found early temporal changes in CBV during radiotherapy to high-grade gliomas.25 These changes were predictive for survival as early as at week 1 of radiotherapy compared with before radiotherapy. Low-grade gliomas with high rCBV values have been associated with more rapid progression and malignant transformation.26 Similar results have been published for the assessment of brain metastases from various primaries that were treated with stereotactic radiosurgery.27 A decrease of CBF within the first six weeks was highly predictive for outcome and treatment response after six months of follow-up. Table 1 provides a list of recent clinical studies using different modes of perfusion MRI to grade brain tumors and monitor clinical and radiographic responses to a variety of treatment modalities.
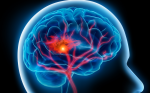
Many have looked into grading gliomas based on their blood supply and CBV.29–31 Most studies were able to correlate increased CBV with higher pathologic tumor grade. An example of a CBV map of a glioblastoma multiforme is shown in Figure 1. However, the level of discrimination for different histologies is too weak to base a differential diagnosis solely on CBV maps. Further clinical applications of perfusion imaging have included a potential role in biopsy guidance and differentiation of radiation necrosis versus tumor recurrence.
T2*-weighted images demonstrate increased rCBV in the right fronto-parietal lobe glioblastoma multiforme.The increased perfusion, as depicted by red and yellow colors can be compared with the relatively normal perfusion of the contralateral side.
New Developments
Most recently, diffusion mapping and tractography of white matter tracts have been found to be a useful adjunct to neurosurgical planning.34 Diffusion tractography is the first imaging modality that allows for in vivo visualization of neural connections and their displacement, for instance by tumors.35 It has also been used for the observation of white matter tract maturation,36 white matter tract diversions by hematomas37 and multiple sclerosis lesions,38 and the reorganization of white matter tracts in blind humans.39 This has great potential to enhance our understanding of how brain tumors interfere with physiological brain function. Diffusion tractography may also prove to be a useful tool for enhancing precision and tissue-sparing with neurosurgical management and radiation therapy.
Conclusions
In summary, perfusion MRI has developed into a very useful and promising adjunct to our current imaging modalities. We are now able to not only visualize anatomical borders, but to observe physiological function within the tumor. It is already possible to monitor treatment effects of both conventional and newer targeted therapies. Further studies are needed to establish the role of perfusion MRI as a surrogate biomarker for outcome, particularly in the field of antiangiogenic therapies. This would allow us to individualize new treatment protocols more effectively. Other new methods, such as diffusion tractography, are promising tools that will provide further insight into the pathophysiology of brain tumors.