Complex regional pain syndrome, type 1 (CRPS1)/reflex sympathetic dystrophy (RSD) is a neuropathic pain disorder,1 with pain reflecting partially damaged nerve fibers.2 It is normally caused by traumatic injury, further aggravated by neuroinflammation.3 The latter contributes to the devastating potential for CRPS1/RSD progressing to a permanent, totally disabling condition.4 Identifying CRPS early, when classical features may be difficult to detect, permits prompt and more efficient efforts at blocking proinflammatory and neuropathic responses. This includes utilizing anti-inflammatory medication and mobilization-focused treatments soon after onset,5 which promotes the greatest clinical benefit and potential for recovery.
There are three essential points to consider in the successful management of people affected by CRPS1/RSD. The first is the awareness and consideration of a CRPS1/RSD diagnosis as early as possible, which includes recognizing skin manifestations; one of the earliest objective findings of this disorder.6 The second is awareness of the variable expression of established diagnostic criteria in the earliest appearance of CRPS. Early symptoms can vary with activity, posture, temperature, humidity and stress.7 This variability can delay proper diagnosis until the condition has progressed to a stage where it can be recognized by the Budapest Criteria diagnostic features.8 The third point is that the nervous system has features of both redundancy and plasticity,9 so efforts at treating this condition always have the potential for a positive outcome. The goal of the present review is to facilitate the identification of early objective markers of CRPS1/RSD with a view to improving its treatment.
Overview of the basic CRPS1/RSD characteristics
CRPS2/causalgia was the first form of this pain disorder characterized,10 and reflects clinically evident damage to a specific nerve. The symptoms and signs of both forms of CRPS1/RSD and CRPS2/causalgia are otherwise identical. Nevertheless, identifiable nerve damage in CRPS2/causalgia provides an anatomical substrate for corroborating, and thus legitimizing, this diagnosis. Consequently, CRPS2/causalgia has been more straightforward to confirm, and therefore, a less controversial diagnosis.
In CRPS1/RSD, the damage affects microscopically small-sized nerve fibers.2 Although this can be proven on biopsy specimens, in clinical practice it is not always possible or practical to biopsy an area in which the patient is experiencing intense pain in order to confirm a diagnosis that can ultimately be clinically established. Typical features of a specific nerve injury are not usually evident under these circumstances. The diagnosis of CRPS1/RSD depends upon awareness of the constellation of clinical features of presentation.
Recent evidence suggests that the clinical progression of CRPS reflects a number of events, with a neuroinflammatory component prominent among them.1,3,11 The transition from acute to chronic pain is arbitrarily defined by pain persisting beyond the normal period of healing, citing periods between 30 days and 6 months.12,13 Prolonged inflammation causes persistent pain and clinical progression to the chronic phase. Identification of this progression, and the role of inflammatory mediators in this process,1 suggest potential therapies. Therefore, the importance of early diagnosis is evident, facilitating prompt treatment and permitting resolution prior to the development of sequelae and spread.14 Delaying diagnosis, and consequently delayed treatment of this neuropathic pain disorder results in a persistent tissue microenvironment which allows the progression of CRPS1/RSD into a chronic pain disorder.1,3,10–12 The consequences are disruption of normal tissue architecture and function, and the capacity of spread to structures beyond the site of origin. Consequently, CRPS1/RSD is often an important potential cause of permanent, total disability.4
Initial efforts in treating CRPS1/RSD are focused on assessing tissue structural integrity, reducing post-traumatic swelling, reducing inflammation, reducing pain, and promoting frequent, repetitive therapeutic movements to restore functional mobility.15 Additional treatment options are available for more advanced forms of CRPS1/RSD, but they all begin with the approach described, and frequently require the addition of more intense forms of intervention.16 One should also be aware that with more intensive therapies there often are more severe side effects.
CRPS1/RSD awareness—diagnostic perceptions and their impact on early diagnosis
There has long been a problem with awareness of the diagnosis of RSD. In part this reflects the stigma and controversy of regional pain occurring in the absence of a validating identifiable nerve lesion. Pain is a symptom. Without objective corroboration, issues of hysteria, somatoform disorder, and secondary gain are often raised. Additionally, diagnostic confusion arose based upon the clinical specialist evaluating the patient. Each medical specialty has defined its own diagnosis for this condition.17 For example, algodystrophy, causalgia (major/minor), post-traumatic pain, shoulder-hand syndrome, and Sudeck’s atrophy are several specialty-based diagnoses. Treatment strategies also vary depending on the approach of the particular specialist. Although RSD was first introduced by Evans in 1946,18 and was an important step in recognizing this condition, it did not improve treatment. It was not until the mid-1980s that it became clear these assorted diagnoses were the same condition, RSD.17
In an effort to further characterize RSD, Bonica identified stages ranging from I–III, which corresponded to acute dystonic and atrophic phases.19,20 This scheme arose from the observation that the course of RSD evolved over time; 1–3 months in stage I, 3–6 months in stage II, >6 months as stage III. It was believed the progression was relentless and predictable. However, there are two points to consider. First, the chances of responding to treatment in stages I and II were better than in stage III. Second, not everybody relentlessly progressed, for reasons that remain unclear at present, and therefore, the staging system is no longer utilized. Differentiating the early treatment-responsive stages from the more advanced treatment-resistant stages was lost in the emphasis on formulating reliable diagnostic criteria.
In 1994, a subcommittee of the International Association for the Study of Pain suggested a name change for the disorder from RSD to CRPS.21 The new name de-emphasized the role of a reflex response, sympathetic nervous system (SNS) involvement, and dystrophic features of the disorder. Instead, emphasis was on a pain syndrome being complex in its diverse expression of symptoms and signs, and its regional distribution rather than being confined to a specific peripheral nerve or nerve root localization.22 Sympathetic nerve blocks had previously been utilized to aid in diagnosis. Unfortunately, there have been individuals with clear clinical signs of CRPS, who failed to achieve pain relief with a technically well-performed sympathetic nerve block. Additional characterization was added by differentiating those who responded and those who did not respond to the block to address this observation. Those who responded were considered to have sympathetically maintained pain,23 while those who failed to respond were labeled as having sympathetically independent pain.24
With a more uniform nomenclature, the next hurdle was differential diagnosis. Diagnoses readily confused with CRPS1/RSD could include those with fairly symmetrical symptoms, such as Raynaud’s syndrome and scleroderma, and possibly diabetic polyneuropathy. Alternatively, conditions with asymmetrical presentations could resemble CRPS, including diabetic neuropathy and post-herpetic neuralgia amongst others. However, these conditions tend to have distinct abnormalities on blood testing and do not have a specific traumatic origin preceding onset.
CRPS1/RSD awareness—elements of CRPS pathogenesis
The primary feature of CRPS reflects a lowered sensory threshold to pain. This change may begin at the peripheral pain receptor (nociceptor), which leads to involvement of the nerve fibers mediating sensation. This includes the smallest diameter c-fibers and Aδ fibers.15 In addition, there are changes that follow within the spinal cord dorsal root entry zone. This reflects the release and action of excitatory neurotransmitters due to the neuroinflammatory status initiated at the nociceptors. The excitatory neurotransmitter N-methyl-D-aspartate (NMDA) receptor is deregulated in this environment. The activity of the NMDA receptor has led to the use of ketamine in an effort to reset this process because of its ability to inhibit the NMDA receptor.16
Vasomotor, sudomotor/edema, sweating, and trophic features are mediated through actions of the SNS. Patterns of blood flow are altered through the sympathetic activity which produces color, temperature, swelling and sweating changes. Trophic effects on skin, hair, and nails are also influenced by the sympathetic activity. There is a trophic link between the cells of the SNS and the sensory dorsal root ganglion cells, nerve growth factor (NGF).25 NGF and its receptor (NGF-R) are typically expressed only during developmental life stages, such as the formative stages of limb development. Expression of NGF and its receptor has a profound impact on both sympathetic and dorsal root ganglion cells during development, until movement is established and then these molecules essentially hibernate. Following injury, both NGF and NGF-R are re-expressed. However, these molecules, and others, can act as proinflammatory mediators, with potentially devastating consequences. Recent efforts have focused on these molecules as potential targets of non-opioid pain management.26,27
Finally, motor function is influenced by aberrant sensory input in CRPS,28 with intense pain limiting movement, and sympathetic dysfunction, such as the fight or flight or the shivering response, each of which impact motor output. It is apparent that there is a complex anatomical and functional substrate through which the clinical features of CRPS are mediated.
The Budapest Criteria and other diagnostic features of CRPS1/RSD
CRPS1/RSD in its most flagrant form is easily recognized. There are four major categories in the Budapest Criteria (Table 1), utilized in order to diagnose CRPS.8 These criteria are widely accepted at this point. They have been under study for over 25 years and have been experimentally validated.

The features grouped within the Budapest Criteria are somewhat discordant. In an effort to be inclusive, temperature and color, swelling and sweating, and motor and trophic symptoms and signs are assessed. Although sensory and motor symptoms and signs may be noted, vasomotor and sudomotor symptoms are often more variable, depending on activity, posture, ambient temperature and/or humidity and stress. Overall, this issue impacts scoring, which can create clinical uncertainty, delaying the diagnosis and deferring the opportunity to treat.
Additionally, not all symptom and sign categories are consistently evident at the onset of CRPS. An inadvertent delay in diagnosis can occur while awaiting fulfillment of the Budapest Criteria. It may be preferable to identify pain (burning, freezing, aching, shooting, stabbing, etc.), swelling (measuring limb circumference), temperature change (measured with an infrared thermometer), sweating, color change (observed in photographs), trophic changes of the skin, hair and/or nails (observed in photographs), and motor changes (altered reflexes, tremor, altered posture, altered motion, weakness) in the context of their onset history and persistence. This information can be utilized in conjunction with interpreting the Budapest Criteria; however, acknowledging the potential for variability by history and on examination. One should ask, “does the preponderance of evidence support the diagnosis of CRPS, or not?”
In order to obtain information relevant to the clinical diagnosis of CRPS, the following nine diagnostic questions are proposed:
- Is the pain out of proportion to the injury?
- Is there a burning or aching quality to the pain?
- Is there associated allodynia (pain from a non-painful stimulus), hyperpathia (exaggerated/prolonged pain to a painful stimulus), or hyperalgesia (increased sensitivity to a painful stimulus)?
- Is there swelling (edema)? Is there sweating?
- Are there associated color or skin temperature changes?
- Is the skin smooth, shiny, or are there pronounced hair follicles?
- Is there increased or decreased hair growth?
- Are the nails cracked and grooved?
- Are there abnormal movements, tone, posture (dystonia), reflexes or weakness?
Ensure structural integrity of tissue before mobilization treatments
Normally, the pain of an injury should steadily lessen with the passage of time. With CRPS, the pain does not dissipate, and typically becomes more intense. Therefore, it is imperative to be certain there is no residual damage to the structural integrity of the affected limb (i.e., broken bone, torn tendon), no ongoing infection and no other underlying pathological process that may be the source of the painful disorder. Diagnostic testing, such as X-ray, triple-phase bone scan, magnetic resonance imaging (MRI), and other related studies can be helpful in confirming the diagnosis of CRPS when they reveal positive results. However, these studies do not consistently yield reproducible CRPS findings, and should not routinely be depended upon as tools to confirm the diagnosis. Therefore, it is wise to only request these studies to assess the structural integrity of the tissue prior to initiating movement-based therapy.
In addition to assessing structural integrity, digital photographs of the limbs, which can conveniently be obtained using the high-resolution camera on a smartphone, provides photos which can be observed at higher magnification. As one of the early objective features of this disorder are skin manifestations,6 photographing from the fingertips to the elbows, and the toes to the knees, can be an important tool to evaluate the hair follicles, which affords an important objective parameter to assess in CRPS.6
Awareness and recognition of symptom variability in early disease stages
Clinical experience has shown that CRPS1/RSD is less likely to respond to treatment in its more advanced disease phases. For this reason, it is pertinent that clinicians are able to recognize the early, variable signs of CRPS1/RSD, which can provide the opportunity to initiate treatment early and facilitate resolution of this disorder, prior to the development of sequelae and spread. Despite the utility of the validated Budapest Criteria for diagnosing CRPS, the issues regarding early diagnosis and spread of CRPS, were not addressed in these criteria. Because the early features frequently are labile, the diagnosis may be delayed until they are more consistently displayed. This diagnostic uncertainty may contribute to a delay in the onset of treatment. Similarly, when CRPS spreads to another limb, findings may also be variable. This too can potentially delay treatment. It deserves repeating that symptoms and signs will commonly vary with activity, posture, ambient temperature and/or humidity and with stress. These issues are not typically addressed in the conduct of a standard neurologic examination, but deserve consideration in the context of CRPS.
It is better to err on the side of treating, while limiting invasive efforts. Early and consistent mobilization provides a better opportunity for a positive outcome. Early success at treatment has important physical and psychological implications. Furthermore, immobilization is recognized as a dominant factor predisposing to development of CRPS. Therefore, a recurrent theme in the treatment of CRPS is early and frequent repetitive mobilization.
Although always characterized by pain, because even minor trauma can precipitate CRPS, its other clinical features may be overlooked. A patient may be doubted because of behavioral issues, or pending litigation, possibly suggesting malingering or a somatoform disorder.29 Complaints of burning or aching pain should always be taken seriously, especially early in their course, when most responsive to treatment. Although other conditions may present in a similar fashion, it is important to raise awareness of CRPS as a potential diagnosis when there is persistent pain out of proportion to an injury, and there is no other condition that can better explain the symptoms.
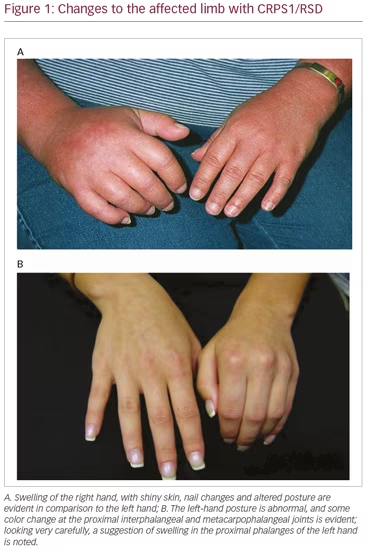
Unfortunately, many clinicians who encounter individuals with early CRPS may have never seen this disorder outside of a publication, if at all. This leaves them uncertain of what is necessary to secure the CRPS diagnosis, which can also delay appropriate treatment. In simplest terms, the characteristic persistent pain following a trauma should always raise suspicion of the diagnosis of CRPS. Furthermore, current awareness of the presenting picture of CRPS is frequently biased to the expectation of encountering a full-blown presentation associated with profound changes in color, temperature, and swelling of an affected limb (Figure 1A). Therefore, accurate early diagnosis requires specific sensitivity and awareness of the potential variability of this condition (Figure 1B).
The sympathetic nervous system in CRPS and its spread to other body parts
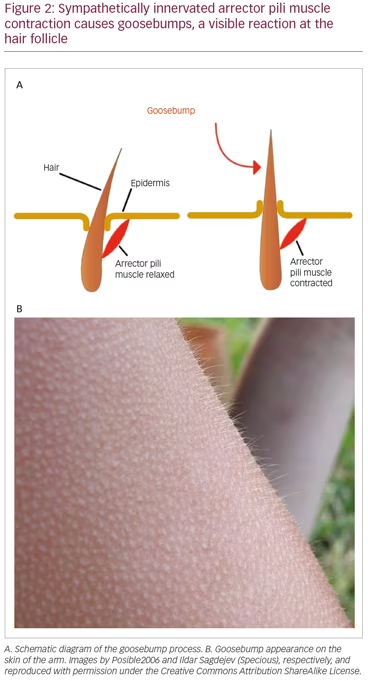
It is apparent that the SNS plays a significant role in producing the initial presenting skin symptoms through localized action.30 The anatomical substrate (Figure 2A) for this is the same as that in the goosebump reflex (Figure 2B), a reflex which has both thermal and emotional triggers, as well as shivering.31 The SNS also plays a significant role in mediating the spread of CRPS to other portions of the body through its network-like organization.6,7 This reflects a critical function of the SNS, which plays a pivotal role in the regulation of blood pressure and core body temperature.32,33 However, the SNS is an important interface between pain signals and the subsequent neuroinflammatory response in CRPS.34–37
It is noteworthy that CRPS symptoms most frequently affect the limbs, and are typically most pronounced at the distal portions of the limbs. This is the consequence of reflex cutaneous vasoconstriction, which functions in response to cold, and peripheral vasoconstriction is initiated distally. This reflex effectively preserves core body temperature longer in cold temperatures, but with the potential for frostbite at the distal extremities. The earliest objective changes in CRPS appear to selectively impact the hair follicle, which has a prominent sympathetic innervation. These early skin changes can be visualized utilizing digital photography. Examples are provided in Figure 3A–C. The stippled-appearing pattern of the skin, with pronounced and hyperpigmented change affecting the hair follicles (Figure 3C), resembles the pattern of goosebumps (Figure 2B).
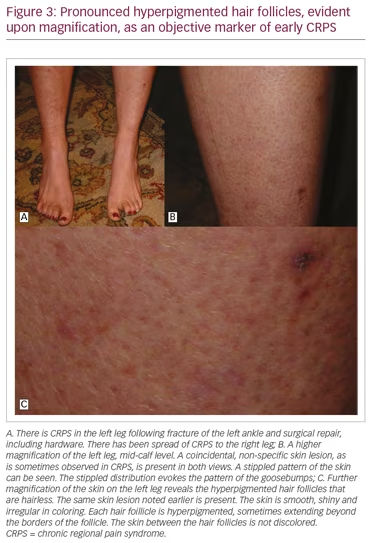
Awareness of this objective finding can facilitate awareness of the early diagnosis of CRPS, when the greatest benefit of movement-based treatment can be obtained. If pain interferes with compliance with movement, there are a variety of treatment options that can be employed.38 If untreated, or even incompletely treated, the severity of the pain from this disorder has driven people to suicidal ideation, and some to suicide.39 This problem is compounded by difficulties in obtaining adequate pain-relieving medications and the stigma associated with their use for pain control. Ironically, while opioids for pain relief are under critical review, cannabinoids in the form of medical marijuana, once feared as a gateway drug, are now receiving increased interest and support in clinical use.40 More studies are warranted to clarify these issues. However, we will now focus on pain management issues.
The natural history and pathophysiology of CRPS1/RSD is generally acknowledged as highly variable from individual to individual and from time to time within any given individual. This disorder can be the cause of significant morbidity and loss of quality of life. It affects mood, cognition, sleep (falling asleep and staying asleep), bladder and bowel function,
self-esteem, job, family, home and includes side effects of medications (such as dry mouth and constipation). There do not appear to be psychological or psychiatric predisposing factors to the development of CRPS1/RSD, although there frequently are consequences of depression and anxiety following its onset.
Therapeutic approach to patients with CRPS—rational polypharmacy
Treatments to address the earliest symptoms, including swelling and aching pain, may utilize steroids.5 More likely, treatments will include acetaminophen or non-steroidal analgesics. On initiating treatment for an early presenting constellation of symptoms, within the first week of symptoms, a short course of methylprednisolone, which affords 6 days of tapering therapy can be beneficial to jump-start treatment (personal experience). This provides 24 mg, 20 mg, 16 mg, 12 mg, 8 mg, and 4 mg of methylprednisolone by mouth. An oral histamine blocker for protecting the stomach, such as 20 mg famotidine, and 0.5 mg lorazepam at bedtime for the 6 days of treatment to reduce jitteriness can make this more tolerable. It is rare that patients require a steroid taper after such a short course of steroids. This treatment protocol is most effective in the earliest phase of CRPS. Therefore, the window of opportunity for this treatment will rapidly close. The steroid works by restricting the impact of the pro-inflammatory factors released as a consequence of the soft tissue injury and to reduce swelling. This facilitates efforts at promoting movement therapy.
Adjunctive treatment for specific CRPS symptoms, such as burning pain and allodynia cannot always be accomplished with a single therapy. Empirically, amitriptyline, nortriptyline, clonidine, gabapentin, levetiracetam, and memantine may individually or in some combination significantly reduce these symptoms. Zonisamide and topiramate are effective for shooting pain. Therefore, it is essential to address the concept of rational polypharmacy in which more than one medication is used at the same time to achieve a better therapeutic goal. Selected additional symptoms have therapeutic options which can provide relief. The pathophysiology of this disorder is complex,12,25,32 and rational polypharmacy acknowledges that issue.
Regardless of the symptom being treated, the focus is on restoring mobility, including repetitive mirror movement-based therapy,41,42 which is not usually possible without significantly reducing pain. Pain intensity does decline with frequent repetition of movements, arbitrarily set at 15 repetitions each time. Additionally, although there is a great desire to avoid the use of opioids because of their stigma and their inherent risks, if non-opioid therapies cannot successfully reduce the pain to a tolerable level, it is imperative to find a way to enable movement. If this requires the use of opioids for this non-malignant disease, it is to be considered.
The next therapeutic group is used to address secondary symptoms of CRPS, such as depression and insomnia or those arising from the impact of the medications used for treating CRPS. This group also includes headache, and bladder, bowel, or sexual dysfunction. A variety of antidepressants are available to choose from, as well as numerous medications that are specifically designed for these selected applications. It is imperative to be aware of potential drug-drug interactions and the side effect profiles of the medications chosen as these new agents are added.
Sometimes a side effect can be therapeutically useful. For example, if muscle spasms and headaches are secondary symptoms requiring treatment, along with difficulty falling asleep and staying asleep, tizanidine, an alpha-2-adrenergic agonist may be quite useful. It is approved for the treatment of painful muscle spasms, but it can be quite helpful in treating headaches as well. Sleepiness is a side effect, but because it has a relatively short half-life, it may be dose adjusted for the individual to help treat all of these symptoms. Once again, combinations of different medications may sometimes be needed in order to provide relief or reduction of symptoms in order to facilitate the movement needed to treat CRPS.
Therapeutic approach to patients with CRPS—movement in warm water therapy
Warm water (aquatic) therapy has many distinct advantages for the person suffering from chronic intractable pain, as long as there are no open wounds which can be compromised by the wet environment. Attention to temperature can avoid issues reflecting temperature sensitivity. The water provides buoyancy for balance and support, and creates muscle resistance to retrain the nervous system. Theoretically, this is the least invasive form of treatment for helping a patient with CRPS experience a return of movement.43 A whirlpool offers the additional advantage of massage, with intensity adjusted by distance from the jets. Nevertheless, the goal of all treatment is adequate pain relief to reach the point of being able to move independently and regularly.
There are two additional points to consider. Pain perception is the consequence of several steps in the processing of ‘pain’ signals from peripheral receptors through perception of pain at the cortex.44–46 These processes include signal transduction, transmission, modulation, and perception. Additionally, excitatory molecules and inflammatory mediators contribute to the phenomena that alter the orderly process of signal processing through peripheral sensitization, wind-up, and central sensitization.47–50 These changes contribute to resistance to movement and a number of movement disorders associated with CRPS.51–54 These dynamic aspects underlying CRPS as it progresses underscore the importance of early intervention.
Conclusion
In conclusion, the key to successful treatment of CRPS is to begin as early as possible, so it does not become a chronic disorder due to secondary changes precipitated by neuroinflammation. This requires awareness of the condition and hypervigilance in recognizing early objective findings of CRPS. A key to awareness is to think ‘CRPS’ whenever the pain following an injury is getting worse, not better, and infection and other structural causes have been ruled out. Hypervigilance in the detection of early CRPS requires appreciation of the variability of the presenting symptoms and signs early in the course of CRPS. Additionally, recognizing the early skin manifestations provides an objective marker of early CRPS. Once underlying structural lesions have been ruled out, it is imperative to make every effort to mobilize the injured region and its mirror-image. Mirror movements, aquatics, and other forms of physical therapy can lead to resolution of this disorder. A variety of medications which target neuroinflammation and either the nociceptors in the skin or the central pain pathways may be needed to facilitate movement therapy. It should be emphasized that their use will not likely produce a totally pain-free state, but the goal should be tolerable pain-reduction. Early diagnosis provides the opportunity for early repetitive mirror movement-based therapy, which in turn provides the opportunity for resolution of the neuroinflammatory cycle that can otherwise lead to a permanent disabling condition, with the potential to spread throughout the body. 













