Neuropathic pain arises from injury to or disease in the somatosensory nervous system.1 Normal sensation and pain sensation are both impaired and patients may present with peculiar painful characteristics such as allodynia and hyperalgesia.2 Central neuropathic pain includes pain from spinal cord injury and demyelinating diseases of the spinal cord, such as multiple sclerosis.1 Lesions or disease of the brain, as in cerebrovascular disease, may lead to post-stroke pain; and neurodegenerative diseases, such as Parkinson’s disease, may also be associated with central neuropathic pain.1 Peripheral neuropathic pain is associated with a variety of conditions, such as diabetes mellitus, peripheral nerve injury, painful radiculopathy, post-amputation pain and postherpetic neuralgia to name a few.1 Types of central and peripheral neuropathic pain conditions encountered clinically are listed in Box 1 and Box 2.
The prevalence of neuropathic pain in the general population is estimated to be 7–10%.3 Pharmacotherapy is often the initial step in treating neuropathic pain.4 International pain organizations, including the International Association for the Study of Pain (IASP), have, over the years, examined the efficacy of pharmacotherapeutic agents to provide guidelines on their use.5,6 Nonetheless, many neuropathic pain conditions are challenging and a significant number of patients may not experience satisfactory pain relief.7 Poorly relieved pain has significant physical, psychological and socioeconomic impact, affecting health-related quality of life.8
This review will briefly discuss interventions that may be considered as next steps when patients fail medical management with oral or topical analgesics for chronic neuropathic pain. These interventions include regional techniques, such as sympathetic blocks, intravenous ketamine infusions and neuromodulation using spinal cord stimulation (SCS) or peripheral nerve stimulation (PNS).
Mechanisms leading to neuropathic pain
Changes in the periphery
When peripheral nerves are injured, spontaneous ectopic discharges occur at the site of the injury, at the neuroma and at the dorsal root ganglion.2 Increased spontaneous activity may also take place in adjacent uninjured nociceptive afferent fibres, influenced by release of nerve growth factor associated with Wallerian degeneration.2,9 There is upregulation of sodium channels, namely Nav 1.7, 1.8.10 In addition, injury evokes an inflammatory response, resulting in the release of chemical mediators such as histamine, neuropeptides and trophic factors.10 These mediators sensitize the peripheral afferent fibres and lower the threshold for firing.2,10 The lowered threshold in ion channels, such as the transient receptor potential channels, contributes to generating and maintaining the increased ectopic activity.2,11
Central sensitization
The increase in ectopic activity in the peripheral afferent fibres and the increase in afferent signalling to the dorsal horn lead to increased excitability in the dorsal horn neurons.11 These neurons lower their threshold, expand their receptive fields and exhibit ‘wind up’, an amplified, prolonged response to repetitive stimulus.12 Complex changes at cellular and molecular levels lead to spinal hyperexcitability.11,13 N-methyl-D-aspartate (NMDA) receptors are activated by the excitatory neurotransmitter glutamate in the presence of injury.14 Consequently, the NMDA receptor plays a major role in pain processing in the spinal cord, whereby receptor activation results in a hyperexcitable state of the nervous system and increased pain.11,14
Central sensitization is also maintained by activities from other cells in the area of injury, namely, white blood cells, T cells and macrophages in the periphery, and glial cells and Schwann cells in the central nervous system (CNS).15 These cells produce cytokines (chemokines, nerve growth factor, interleukins) in response to injury and inflammation. Ultimately these events lead to pain hypersensitivity and persistent neuropathic pain.16 Other mechanisms that may contribute to central sensitization include loss of GABAergic interneurons in the dorsal horn and changes in descending modulation which, in turn, alter the balance between inhibition and excitatory signalling.11
Sympathetically maintained pain
The coupling between the sympathetic and somatosensory nervous system is thought to contribute to pain seen in complex regional pain syndrome (CRPS), in peripheral nerve injury and in chronic painful conditions, such as acute herpes zoster and postherpetic neuralgia.17 The abnormal coupling may occur at the site of nerve injury, the neuroma or in the dorsal root ganglion.18 Although the mechanism of sympathetically maintained pain is not entirely clear, it may be associated with the expression of alpha adrenoreceptors in dorsal root ganglion neurons and the abnormal sprouting of sympathetic efferent nerves, and may manifest as allodynia, hyperalgesia and autonomic changes such as vasoconstriction and hyperhidrosis.19 Patients with CRPS who respond with greater than 50% improvement in pain relief after a sympathetic block may be said to have sympathetically maintained pain, whereas those with less than 50% improvement may have sympathetic independent pain, although some pain component from sympathetic activity in the latter is not entirely ruled out.19
Rational approach to interventions when conventional pharmacotherapy fails
What are some other interventions that one might consider for chronic neuropathic pain after failed medical and failed non-pharmacological, non-invasive management? Regional techniques, such as sympathetic blocks, are widely used in clinical practice as the next step, particularly in patients with CRPS.20,21 Intravenous ketamine infusions for chronic neuropathic conditions may be considered concurrently or after sympathetic blocks have been tried and have failed to provide adequate or sustained relief.22 In patients who continue to experience refractory pain, neuromodulation using SCS and PNS may be considered as next steps.23
The placement of the lead in SCS is, comparatively speaking, a more invasive technique with potential risks and serious adverse effects, even though these are rare.24 It is reasonable, therefore, to consider SCS after regional techniques and infusions have been tried and failed. New developments in technology delivering PNS are less invasive and employ a percutaneous approach to placement of the stimulating lead with ultrasound guidance,25 as opposed to placement with surgical dissection.26 These technical advances have generated renewed interest for PNS and with this in mind, it appears reasonable to consider PNS earlier in the treatment algorithm rather than later as one would with SCS, in cases of intractable neuropathic pain.25,26
The following discussion will address these interventions, namely, sympathetic blocks, ketamine infusions and neuromodulation using SCS and PNS as options in patients whose pharmacotherapy has failed.
Sympathetic blocks
Sympathetic blocks commonly performed for neuropathic pain conditions include the stellate ganglion blocks for upper extremity pain and lumbar sympathetic blocks for lower extremity pain.27 Other sympathetic blocks include the celiac plexus blocks for abdominal pain; superior hypogastric blocks for pelvic pain; and ganglion impar blocks for perineal, pelvic and visceral pain.27 These interventions are commonly carried out using fluoroscopic guidance,20,21 with the exception of stellate ganglion block where the use of ultrasound guidance is preferred by some practitioners.28 The stellate ganglion and the lumbar sympathetic blocks are commonly utilized in clinical practice for neuropathic pain in upper and lower extremities,20,21 and will be briefly described.
Stellate ganglion block
The stellate ganglion is anatomically comprised of the fusion of the inferior cervical ganglion and first thoracic ganglion at the level of C7 (Figure 1).28 The stellate ganglion block is used predominantly to treat pain in the upper extremity in patients with CRPS,20,21 and has also been reported for use in acute herpes zoster, postherpetic neuralgia, phantom limb pain and facial pain.23,27 Patients undergoing a stellate ganglion block may experience Horner’s syndrome as a result of blocking the upper and middle cervical ganglia supplying the head and neck region.29 Common manifestations of Horner’s syndrome include ptosis, miosis and enophthalmos. A successful stellate ganglion block may be indicated by an increase in temperature,18 changes in skin conductance, and increase in blood flow in upper extremity as measured by pulse wave Doppler.30
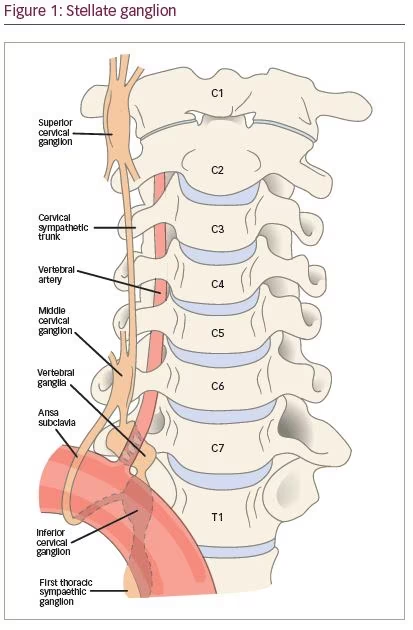
Patients experiencing analgesia after sympathetic blocks may have sympathetically maintained pain, in which case, the sympathetic blocks may be construed as both diagnostic and therapeutic.18,19 Anatomical variation resulting in alternate sympathetic pathways to the brachial plexus through the “nerves of Kuntz” may account for the incomplete sympathectomy with stellate ganglion blocks or when surgical sympathectomy is performed.31 Risks with stellate ganglion block include local anaesthetic toxicity with intravascular injection, haematoma, infection, oesophageal trauma, thyroid trauma and pneumothorax.29
Lumbar sympathetic block
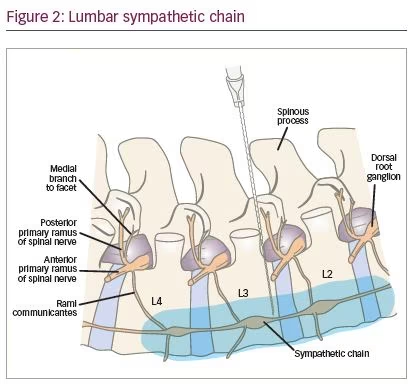
The lumbar sympathetic chain lies in a fascial plane on either side of the lumbar vertebral bodies on the anterolateral border from the second to the fourth lumbar vertebrae, and contain preganglionic fibres arising from T10 to L3 (Figure 2).29 Most of the sympathetic innervation to the lower extremities courses through the second and third sympathetic ganglia.28 Just as stellate ganglion blocks are done for upper extremity pain, the lumbar sympathetic block is often carried out for lower extremity pain, in particular, for patients with CRPS in the lower extremity, for pain in disease states associated with vascular insufficiency, as well as for ischaemic pain.27,29 Risks associated with the lumbar sympathetic block include local anaesthetic toxicity, potential trauma to somatic nerves and infection.29
Who may benefit from sympathetic blocks?
Patients with chronic neuropathic pain, such as CRPS, acute herpes zoster or postherpetic neuralgia, or those who exhibit or report associated symptoms of sympathetically maintained pain, may benefit from a trial of sympathetic blocks.23 These blocks are often utilized in clinical practice as the first interventional approach after failed medical treatment, especially in patients with CRPS.21
The evidence for sympathetic blocks is limited which may be attributed, in part, to the fact that pain mechanisms related to the sympathetic nervous system are not entirely understood.23 Systematic reviews on local anaesthetic sympathetic blocks for CRPS find the evidence limited due to lack of high-quality studies and the small sample sizes in many studies.32,33 Even so, it is reasonable to consider sympathetic blocks as a treatment option, particularly in patients with CRPS early on in the disease process,23 before more invasive alternate treatment options, such as SCS, are considered.
In patients with acute herpes zoster, sympathetic blocks may help reduce the duration of pain.17 A small, prospective, randomized controlled trial studied 20 patients with acute herpes zoster.34 Nine out of ten patients who received local anaesthetic sympathetic blocks had no pain after a series of four blocks, compared with the control group. Retrospective studies looking at larger numbers also found sympathetic blocks reduced pain in a significant number of the patients.35–37 Some studies indicate that the sympathetic blocks in acute herpes zoster may also reduce the occurrence of postherpetic neuralgia, although this remains controversial.37,38 It is unclear if the sympathetic nervous system plays a significant role in the pain mechanism in postherpetic neuralgia, since sympathetic blocks do not appear to be effective in patients with long standing postherpetic neuralgia.17,23
Most of the evidence for sympathetic blocks is found in case series or case reports, with few randomized placebo-controlled trials. Despite moderate and low-quality evidence the grade of recommendation with sympathetic blocks is strong.27 Many of these procedures help reduce pain significantly when pain is refractory to medical treatment, and the benefits often outweigh the risks when performed by trained physicians. The sympathetic blocks are carried out using local anaesthetics and provide relief of variable duration. It remains unclear as to how often one should repeat the sympathetic blocks. Surveys of pain practitioners, in both academic and private practice, show a variation in their assessments regarding the frequency of blocks.21
Sedation during procedures
Some patients are anxious of undergoing procedures while awake, and are uncomfortable despite local anaesthetic infiltration. Sedation during interventional procedures may help allay anxiety, improve patient cooperation by decreasing unwanted movement, and enhance patient experience and satisfaction. However, concerns with increased sedation and inability to provide feedback may pose significant risks.39 For instance, in performing a stellate ganglion block, placement of the needle on a peripheral nerve or in a vascular structure may result in sensory or motor block or seizure from local anaesthetic toxicity. In a prospective study of 155 patients, the majority of the patients surveyed did not report severe procedure-related pain.40 However, the authors did report a small group of patients who seemed to experience severe procedure-related pain. This was not correlated with age, gender or ethnicity. The decision on sedation is best arrived at conjointly between patient and physician on a case-by-case basis after a thorough discussion of the risks of the procedure alongside the risks of sedation.39,40
N-methyl-D-aspartate receptor antagonists
NMDA receptors are activated by the excitatory neurotransmitter glutamate in the presence of tissue injury.11 Consequently, the NMDA receptor plays a major role in pain processing in the spinal cord, whereby receptor activation results in a hyperexcitable state of the nervous system and increased pain.14 NMDA antagonists may alleviate pain by the inhibition of central sensitization.11 The NMDA receptor is a ligand-gated ion channel permeable to calcium, potassium and sodium. At resting membrane potential, the NMDA receptor is blocked by a magnesium ion which is removed on depolarization allowing glutamate to activate the receptor.41 The NMDA receptor is composed of several subunits. Some of these subunits are involved with CNS function; therefore, an NMDA antagonist may produce undesirable psychotomimetic effects, memory impairment, ataxia and uncoordinated motor function.14
Ketamine is a non-competitive antagonist that binds to the phencyclidine binding site of the NMDA receptor.41 It is available as racemic ketamine which contains equimolar amounts of S (+) and R (-) and as the S (+) stereoisomer which is twice as potent. The S (+) ketamine has four times greater affinity for the NMDA receptor than the R (-) ketamine.41 Ketamine has an elimination half-life of 80–180 minutes. The metabolite nor-ketamine is one-third as potent and has a longer half-life and may contribute to the prolonged analgesic action of ketamine.41
How may ketamine be helpful in neuropathic pain?
Ketamine is an anaesthetic agent that has been in clinical use for the past five decades; however, in the past several decades, there has been growing interest in the role of ketamine as an analgesic, in particular, for chronic neuropathic conditions.42,43 This is primarily due to the action of ketamine as an NMDA antagonist in addition to other antinociceptive actions which include interaction with opioid receptors, activation of descending modulatory pathways, anti-inflammatory actions and prevention of hyperalgesia and tolerance.43
Several review articles have reported use of ketamine in chronic neuropathic conditions although it is noted that many studies have limitations related to design, blinding and small numbers.42,44–46 Moderate evidence supports use of ketamine in CRPS.22 The evidence, however, is weak for other neuropathic conditions, including spinal cord injury pain, fibromyalgia, postherpetic neuralgia, phantom limb pain, mixed neuropathic pain, headache, spinal pain and ischaemic pain.22
For patients with CRPS, two randomized, double-blind,
placebo-controlled studies using ketamine stand out. Sigtermans et al. randomized 60 patients diagnosed with CRPS type 1 based on IASP criteria into two groups of 30 patients each: one group received low dose ketamine infusions, the other saline as placebo.47 The infusions were given for over 4 days and patients followed for 12 weeks. Pain scores in the ketamine group were significantly lower until week 12 and the side effects were tolerable. In another randomized, double-blind, placebo-controlled trial, Schwartzman et al. studied 20 patients with CRPS who received outpatient ketamine infusions for 10 days, each with an infusion of 4 hours duration, and tracked patients for 12 weeks. The ketamine group had significant decreases in several pain parameters and did not suffer significant adverse effects.48
What are the issues with ketamine?
Ketamine may be associated with undesirable side effects which are mostly dose dependent. CNS side effects include hallucinations, altered sense of perception and panic attacks.44 Some may experience nausea, headache, dizziness, hypertension and tachycardia. There may be associated elevated hepatic enzymes with repeated use.49 Recreational users or abusers may experience bladder symptoms such as dysuria, haematuria and abdominal cramps. Severe psychological effects include schizophrenia-like symptoms and a sense of impending doom as in near death experience.44
Ketamine may be helpful in managing pain in some patients with intractable chronic neuropathic pain, particularly in cases where nothing else has worked. However, the evidence for use of ketamine in many neuropathic conditions, except for CRPS, is weak.22 In general the studies cited are small and there is difficulty in blinding. There is more work to be done to determine optimal dosing, duration of treatment, long-term adverse effects with repeat treatments, and outcome assessments.22,45
Neuromodulation
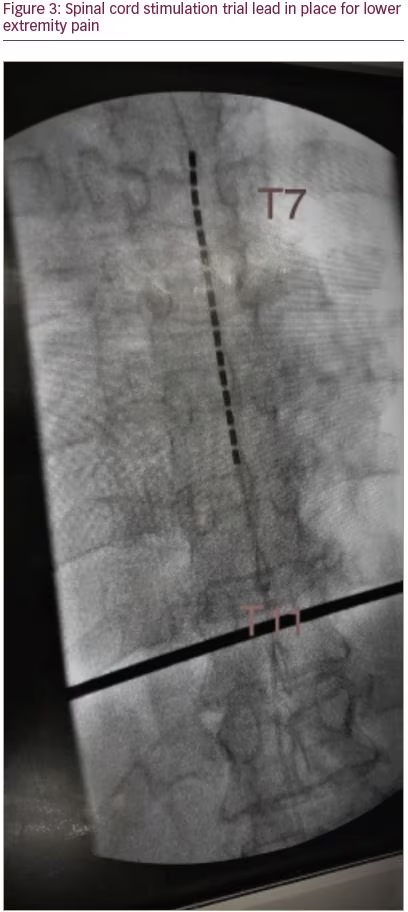
The use of electrical stimulation for pain relief is not a new concept. Reportedly, electric fish were used in ancient Egypt for treating chronic unbearable pain.50 The gate control theory is referred to as one mechanism for pain relief with this modality.51 In this theory, stimulation of non-nociceptive afferent A-beta fibres inhibits the nociceptive afferent fibres, the A-delta and C fibres, by activating inhibitory interneurons in the dorsal horn of the spinal cord.51 Analgesia may also be attained by peripheral stimulation, such as in transcutaneous electrical nerve stimulation, and in PNS,25 or by stimulation of the posterior column of the spinal cord as in SCS (Figure 3).51,52 Both SCS and PNS may be useful in producing a longer duration of relief or sustained relief.26,52
Spinal cord stimulation
SCS has been shown to be effective in refractory neuropathic pain, such as lumbar radicular pain in patients who have failed back surgery.52 One of the mechanisms of pain relief using SCS in neuropathic pain is thought to be related to the decreased excitability of spinal cord neurons as a result of decreased temporal summation.53 Temporal summation is an increased pain response to a prolonged painful stimulation, similar to the concept of ‘wind up’.53 Therefore, the decrease in neuropathic pain may be related to the effect of SCS on central sensitization.12
Over the years technology for SCS has advanced such that the stimulation, commonly a tonic stimulation, can now be delivered as a ‘burst’ stimulation or as a high frequency stimulation without sensory paresthesia.54 Neurostimulation may also be targeted at the dorsal root ganglion.16,55 Studies are ongoing to determine comparative efficacies of these various stimulation patterns. Serious complications are rare in SCS and may involve structures in the neuraxis. These include nerve injury, epidural haematoma, abscess, post-dural puncture headache, wound infection and lead migration.24 Another review of the literature concurs that serious neurological adverse events are rare and report that most complications appear related to the hardware or device.56
Who may benefit from spinal cord stimulation?
Studies and multicentre controlled trials have shown that SCS is helpful in conditions with neuropathic pain.57,58 Common indications for SCS include chronic neuropathic pain and radiculopathies of the trunk or extremities, CRPS type 1 and 2, post laminectomy syndromes, refractory pain in postherpetic neuralgia and spinal cord injury.59,60 In a randomized controlled trial by North et al.,50 patients who failed back surgery and had intractable neuropathic pain in a lower extremity were randomized to either reoperation or to a trial of SCS.57 If the initial treatment did not decrease pain by 50% or more, the patients were allowed to cross over to the other treatment group after 6 months. The crossover rate in the patients receiving SCS (5 out of 24) was significantly lower than in patients who had reoperation (14 out of 26). At a 3-year follow-up, 45 patients out of the 50 were evaluated, and those who had the SCS reported more effective pain control.
In a multicentre randomized controlled trial, 100 patients who failed back surgery and had predominantly neuropathic pain in the lower extremity were randomized to conventional medical management only (CMM) or CMM with SCS.58 The primary outcome was ≥50% relief, and patients were permitted to cross over at 6 months. In the SCS group, 48% achieved the primary outcome compared to 9% of CMM group at 6 months. Between 6 months and 1 year, five SCS patients crossed over to CMM and 32 CMM patients crossed to SCS. However, it was noted that at 12 months 27% of SCS patients had complications related to the device.
Patients with CRPS may undergo sympathetic blocks and ketamine infusions as first interventions after failed conventional pharmacotherapy. If pain relief is inadequate, these patients may consider a more invasive option, such as SCS. In a randomized controlled trial by Kemler et al.,54 patients with CRPS type 1 were randomized to SCS trial and physical therapy or to physical therapy alone.61 Two-thirds of the patients given SCS received a permanent implant. At 6 months, 39% of patients in the SCS group reported that their pain had “much improved” compared with 6% in control group. At 2 years, the follow-up evaluation showed that SCS continued to be effective.62 Long-term evaluations at 3–5 years did not show differences between the two groups.63 In another prospective trial, 29 patients with CRPS type 1, who had responded briefly to sympathetic blocks, were treated with SCS.64 In a follow-up period of up to 3 years, SCS with physical therapy decreased pain and improved function and quality of life.
Harke et al. looked at the effectiveness of SCS in 28 patients with postherpetic neuralgia for over 2 years in a prospective case series.65 They reported significant pain relief in 23 patients and significant decrease in pain disability index. North et al. prospectively followed 62 patients (with failed back, spinal cord injury or peripheral neuropathic pain) with SCS treatment.66 The treatment was successful in 40% of those with spinal cord injury, with at least 50% relief for 2 years. The authors noted that patients with spinal cord injury with segmental pain at or below the level of injury were successful with SCS, and those with diffuse pain from spinal cord injury were not successfully treated with SCS.
Peripheral nerve stimulation
SCS is often identified with the use of neurostimulation for chronic refractory neuropathic pain. However, PNS, where a stimulating lead is placed close to a peripheral nerve to target a specific area of pain,25 is becoming more widely considered as another option, using neuromodulation for treatment of neuropathic pain in the extremities and the trunk, with a moderate level of evidence for treating peripheral mononeuropathic pain (Figure 4).67
Peripheral nerve stimulation: evidence and forward-looking perspective
In past years, PNS was an invasive procedure necessitating surgical dissection to place a stimulating electrode close to the targeted nerve.68 With newer technology the stimulating lead may be placed percutaneously using ultrasound guidance.68,69 The PNS systems available include those that are permanently implanted after a successful trial, in a similar way to the procedure for SCS.69 In a prospective, multicentre, randomized, double-blind, partial crossover trial, Deer et al. assessed the safety and efficacy of this technique.70 Ninety-four patients with intractable pain of peripheral nerve origin were randomized to a treatment (n=45) or control (n=49) group. At 3 months, the decrease in pain was statistically significant compared to baseline in the active stimulation group. Patients were followed-up for up to 1 year and no serious adverse events were reported.
With technological advances, there is now a novel system that is approved by the US Food and Drug Administration, in which the stimulating lead is placed percutaneously and left in place for 60 days, after which, it is removed.71 In a multicentre randomized, controlled trial, patients with chronic amputation pain were randomized to 8 weeks’ PNS (group 1) or 4 weeks’ placebo and 4 weeks PNS (group 2).71 The percutaneous leads were placed at the femoral and sciatic nerves using ultrasound guidance. At 12 months, 67% in group 1 reported ≥50% reduction in pain compared to 0% in group 2. In addition, 56% in group 1 reported ≥50% decrease in pain interference compared to 15% in group 2. There was also significantly less depression in group 1 at 12 months compared with group 2 at crossover. This study suggests that the PNS intervention was efficacious in reducing chronic post-amputation pain with sustained relief and improved function at 12 months. There were no serious adverse side effects reported. There was no need for a permanently implanted system as the leads were removed after 60 days. There were no serious adverse side effects reported.71 Nerve injury and infection associated with PNS are rare.56,71 As PNS technology advances, we anticipate more well-designed clinical trials that will help guide the application and patient selection for this modality.
Discussion
The management of chronic neuropathic pain is challenging. Conventional first-line treatment includes pharmacotherapy, but not all patients respond with optimal or adequate pain relief. Sympathetic blocks are commonly performed in clinical practice, particularly for neuropathic pain in extremities. Ketamine infusions may be tried concurrent with other treatments in patients with intractable chronic neuropathic pain, although the evidence is strongest in patients with CRPS. Newer technology with PNS allows a less-invasive approach, and this option may be considered, if indicated, earlier in the treatment plan rather than later. More invasive options, such as SCS, may be considered when less invasive options have been exhausted.
Although there is the potential for serious adverse effects for some interventions, the risk of serious complications is rare and can often be mitigated by careful patient selection, patient education and risk-avoidance techniques. The evidence for many regional and interventional techniques, including ketamine infusions, varies across the board. However, for patients who suffer intractable chronic neuropathic pain, these interventions may provide much needed options for treating their pain, and are therefore helpful additions to the therapeutic armamentarium for these challenging patients.













