Modern anaesthetic practice requires the anaesthesiologist to care for extremely sick patients while negotiating the intricacies of myriad procedural environments. One such unique environment that is becoming increasingly important for contemporary anaesthesiologists to be familiar with is that of the intra-operative magnetic resonance imaging (ioMRI) suite.1,2
ioMRI is an evolving technology that offers precise intra-cranial lesion localisation and intra-operative navigation by combining the highresolution imaging capabilities of MRI with an operative suite. When ioMRI is combined with other, more conventional, intra-operative navigation strategies, it has the ability to provide increased precision of navigation and aid in resection of a variety of intra-cranial lesions.3–6 There are a number of important considerations when caring for patients in this environment that are quite different from any other perioperative environment.7 As these types of operative suites proliferate, it is becoming increasingly important for anaesthesiologists to become familiar with the unique aspects of delivering safe, effective perioperative care for patients undergoing ioMRI-guided neurosurgical procedures. This review will discuss considerations when caring for patients in an ioMRI suite.
History
ioMRI was developed in the 1990s in an attempt to improve intraoperative navigation and have a real-time assessment of the extent of resection during intra-cranial neurosurgical procedures.8 Prior to the development of ioMRI, a variety of stereotactic navigational systems had been utilised to improve localisation and precision of resection. These techniques employed either frame-based or frameless systems. All these systems involved triangulating a number of points over multiplanar images of the head acquired preoperatively.
The fundamental concern that is common to both types of systems is their reliance on the preoperative imaging. It must be noted that these baseline images occur before positioning for surgery, opening of the craniotomy, loss of blood and cerebrospinal fluid (CSF) and any resection. Thus, the accuracy of these systems can be significantly affected by brain shift, which refers to the intra-operative movement of intra-cranial anatomic structures resulting from a variety of actions including position changes, CSF egress and mass resection.9 The actual amount of brain shift can vary depending on the tissue type of the lesion, patient positioning, size of craniotomy, CSF loss and volume of tissue resected.10 Thus, as the surgical procedure goes on, the accuracy of these types of navigation systems erodes over time. In an effort to address these concerns, the world’s first ioMRI was developed in 1994. Subsequently, it was demonstrated that ioMRI can compensate for brain shift by allowing these navigation systems to be updated as well as giving a real-time assessment of the shifting intra-cranial anatomy during the procedure.10
Types of Systems
There are essentially three types of ioMRI systems in use. They consist of the original type of open system with a stationary magnet and a stationary patient as well as the two more common types in contemporary use – stationary magnet/movable patient and movable magnet/stationary patient. Each system has its own advantages and disadvantages.
The open magnet is the easiest to obtain imaging during surgery, as the patient is physically in the bore the entire procedure.8 However, performing the operation within the high-strength magnetic field also presents several problems. First, there is extremely limited access to the patient for both the surgeon and the anaesthesiologist in the event of an untoward event. Second, because of the magnetic field, there are limits on the specific equipment that can be used intraoperatively. This means that conventional surgical instruments including drills and microscopes cannot be used.11 Although MRIcompatible versions do exist, their quality is considered generally to be not as good as conventional instruments.12 It should be mentioned that this also affects the use of other monitoring equipment, such as electroencephalographic leads and monitors, and, consequently, these types of monitors may be contraindicated in such an environment.11
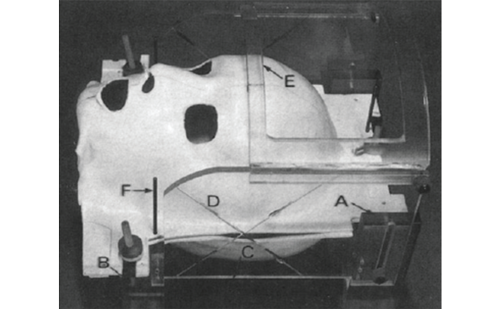
As a consequence of the significant limitations the older-style ioMRI systems imposed on the surgical procedure itself, systems have evolved to alleviate these concerns. The most common type of these newer systems involves a movable magnet and stationary patient. These types of configurations offer several advantages over ‘open’ configurations (see Figure 1).13 However, one drawback to systems that allow for MRI incompatible surgical equipment to be used is that intra-operative image acquisition can only occur after the patient has been placed within the magnet, which requires additional time.14 If this process is initiated prior to closure of the craniotomy, the sterile field will need to be maintained. This can be accomplished by placing sterile drapes over the patient and the open cranium. In our practices we perform extra instrument counts prior to moving the magnet into position to account for MRI unsafe instruments and equipment used during the resection. Despite these concerns, these systems offer some significant advantages. First, the ioMRI suite can be configured to function, in many ways, as a normal operating room. There is greater access to the patient for both the surgeon and anaesthesiologist and normal surgical instruments can be utilised – regular microscopes, drills, retractors, etc. The cost of such a suite can be prohibitive, as these systems require rooms with specialised MRI shielding because of the associated high-strength magnetic fields.
The relative advantages and disadvantages of a stationary magnet/ movable patient system are similar to those for a movable magnet/stationary patient. One potentially beneficial difference with utilising a movable patient platform is the opportunity to include multimodal imaging all in a single operative suite such as positron emission tomography and biplanar fluoroscopy. The disadvantages of this arrangement are also similar and relate largely to the safe docking of the anaesthetised patient within the magnet bore, inherent issues with MRI safe physiological monitors and need for an MRI-compatible anaesthesia machine as the high-strength magnetic field moves to encompass the patient. Despite these concerns, some centres are using these systems with great success.15
Magnetic Resonance Imaging Safety Zones
In order to deliver safe patient care in ioMRI, it is necessary to understand the concept of safety zones that are common to all MRI environments. The American College of Radiology (ACR) has divided the MRI area into four zones to create a common nomenclature and enhance safety in these environments.16,17 Familiarity with this concept is important in order to design processes that facilitate patient care at each juncture of patient interaction in ioMRI. Table 1 provides a description and location of these safety zones within any MRI environment.
Awake Craniotomies in Intra-operative Magnetic Resonance Imaging
The advantages of performing awake craniotomies have been well documented especially when it applies to removing intra-cranial tumours or lesions affecting the eloquent areas.18,19 The benefits of combining awake craniotomy techniques with the intra-operative MRI have also been described.20,21 Once cortical mapping is complete and resection concludes, the equipment used to facilitate the mapping needs to be accurately accounted for and removed from the room prior to deploying the magnet. Slight modifications to the draping technique are necessary to maintain sterility and also avoid claustrophobia while allowing spontaneous respiration in the awake or lightly sedated patient (see Figure 2). Sedation during the MRI scan can be provided using a variety of drugs depending on the individual practitioner’s comfort. Dexmedetomidine, alone or in combination with titrated opioids, can provide adequate sedation during imaging.
Protocols and Checklists
Although utilised by anaesthesiologists for decades, checklists have recently gained widespread popularity in a variety of other disciplines and patient care settings as a tool to help improve the safety and quality of care delivery. Historically, checklists have played a major role in some of the more significant successes achieved in patient safety.22 The ioMRI environment offers a perfect opportunity to utilise checklists and protocols to promote safety. A collaborative, multidisciplinary approach between MR technologists, nurses, surgeons and anaesthesiologists is important in designing protocols and checklists with safety as a central theme. This is especially important when MRI unsafe or conditional equipment is utilised. MRI unsafe and conditional equipment is routinely used during non-imaging portions of the case when the MRI unit is safely garaged and the patient is in Zone III. Checklists provide a systematic method for accounting for such devices. The checklists include tasks to be completed by all members of the team including the anaesthesiologists, surgeons, nurses and MR technologists prior to deployment of the magnet.
Limitations of Intra-operative Magnetic Resonance Imaging
Despite its purported advantages, there are some significant limitations to ioMRI in a variety of settings. These limitations can be best understood when divided into those involving the equipment, the patient themselves and the overall environment. Finally, no technological advance in modern medicine can be evaluated properly unless some mention of cost of the device enters the discussion.
Equipment Concerns
When discussing implantable devices and medical equipment in the MRI environment, certain nomenclature describes their relative safety. MRI safe means that the item poses no risk in such an environment. MRI unsafe means that the item poses a hazard in all MRI environments and is thus contraindicated within Zone IV. MRI conditional means that a given item has been demonstrated to pose no known hazards in a specified MRI environment with particular conditions of use. An example of this is an MRI conditional anaesthesia machine that is conditional to 100 Gauss in a 1.5 T magnet. Beyond that field strength (e.g. 3 Tesla), the item may be unsafe.23
While newer ioMRI systems allow the use of conventional surgical equipment, the same cannot be said for anaesthesia equipment. At the time of imaging, surgical equipment and instruments can be accounted for and moved out of the high-strength magnetic field. At this time, the patient may still have an open surgical wound and will still require maintenance of general anaesthesia. Thus, the anaesthesiologist will need to utilise many MRI-compatible itemssuch as the anaesthesia machine, physiological monitors and infusion pumps. In particular, MRI-safe pulse oximeters, end-tidal gas analysers, electrocardiogram, blood pressure (both invasive and noninvasive) and temperature monitoring must be utilised. Although other equipment can be safely moved outside of the 5 Gauss line when the patient is within the high-strength magnetic field, the anaesthetic must continue. This necessitates using such equipment so that:
- the patient does not suffer an injury (such as a burn from MRIunsafe leads);
- there are no inadvertent ferrous objects that become projectiles in the high-strength field; and
- the monitoring equipment, pumps and machine do not interfere with image quality.
Unfortunately, there are a number of pieces of critical and necessary equipment that do not have MRI safe or conditional analogues.8,24 Among these items are defibrillators, fluid-warming devices, forced air-warming devices, Doppler ultrasound machines, peripheral nerve stimulators and inexpensive core temperature probes. In modern anaesthetic practice, the use of such devices has become standard of care in many settings. Thus, the challenge then becomes how to use such pieces of equipment or have them immediately available without compromising patient and staff safety. In our experience, a collaborative and multidisciplinary approach works best in this situation. Our institutions routinely employ such MRI-unsafe equipment during non-imaging portions of the procedure, when the MRI unit is located in the garage and the patient is in Zone III. In preparation for magnet deployment, all MRI-unsafe components listed above are removed outside the 5 Gauss line. Figure 3 illustrates the relative location of equipment and Zone IV with the garaged magnet (see Figure 3A) and the magnet deployed in position for imaging (see Figure 3B). We have found that the utilisation of safety checklists enhances the ease of accounting for removal of such devices. Our checklist includes all items and matters that affect anaesthesiologists, surgeons and nurses and the entire operating room (OR) pauses to ensure all items are accounted for before the magnet is brought into position around the patient. As a final safety measure, a number of MRI unsafe pieces of equipment in the suite are tethered to the wall with cables to prevent them from ever being inadvertently moved within the 5 Gauss line.
A variety of neuromonitoring devices, such as needle electrodes used in motor and sensory evoked potential monitoring, can present difficulty as they create potential hazards if they are not removed prior to imaging.25 These types of monitors may be safely used in this room as long as meticulous accounting of each lead is completed before the magnet enters the operating room. This is another opportunity to use a checklist approach to enhance safety.
In addition to the anaesthesia and surgical equipment, other devices that are required to be MRI compatible can create problems. MRI compatible head-pinning systems need to be employed. Although these systems allow the use of head fixation in an ioMRI environment, numerous problems exist including poor mobility of the device at its various joints, difficulties with proper pin placement, difficulties with proper pin seating in the skull and device durability issues. Finally, some radiofrequency head coils needed to facilitate image acquisition can create challenges for the anaesthesiologist including limited access to the patient’s airway (especially in a prone patient) (see Figure 4).
Patient Concerns
Patients who have certain implantable devices such as defibrillators or pacemakers should not enter the MR environment because they could potentially suffer injury or death.26–28 There are several other implanted devices that are more controversial, including vagal nerve stimulators29 and cochlear implants.30 The most important intervention the anaesthesiologist can make is to have conversations with the institutional MRI safety officer prior to scheduling patients with questionable implantable devices in an ioMRI suite.
The ioMRI suite is a unique, and potentially patient-unfriendly, environment in which to perform surgical procedures. The magnet must be supercooled with either liquid helium or nitrogen to optimise the magnetic field creating a limited ability to alter room temperature. This can have significant consequences when caring for small children, especially neonates, in this cold environment.31 Babies, and particularly neonates, have a limited ability to self-regulate temperature, especially under anaesthesia. Thus, exposure to a cold environment can result in significant hypothermia and its attendant consequences. Active heating using forced hot air warmers or fluid warmers is essential to maintain normothermia in this environment. These devices (MRI unsafe) must be discontinued during imaging portions of the procedure when the patient is placed in Zone IV. It is useful to keep the patient covered with the sterile drapes during the imaging phase to maintain temperature.
In addition to concerns with patients at the smaller end of the size spectrum, larger patients present their own set of issues. When determining the limits of size of patient that can safely undergo intra-operative imaging, the size of the bore of the magnet will be the limiting factor. It is crucial to note that while a patient may fit into the bores of some magnets in diagnostic settings, that same patient may not necessarily fit into the ioMRI magnet due to a potentially different bore size, the additional burden of drapes to maintain sterility and surgical positioning concerns. Pressure ulcers with localised ischaemic tissue injury as well as compression resulting in peripheral nerve injury can result from direct skin contact with the operating room table, other equipment such as imaging coils, and the inner bore of the magnet. It is useful to have a template of the size of the bore of the ioMRI magnet to trial the patient before deploying the magnet. Padding likely pressure points may ameliorate these factors. Ultimately, proper patient selection is best.
Cost Concerns
There are legitimate concerns about the cost of setting up an ioMRI system both from the initial financial investment and the increased duration of the ioMRI cases. Each MRI sequence may add 30 minutes to 1 hour to the duration of surgery. A recent study found the average length of surgery was increased by 1 hour 47 minutes when comparing ioMRI with conventional non-ioMRI neurosurgical resection of intracranial lesions. In 42 % of the cases, imaging led to further tissue resection. Further, there was an increase in early reoperation as defined as within 2 weeks of surgery at 7.7 % in the non-ioMRI group compared with 0 % in the ioMRI group. Aside from increasing operative costs, the increased duration of surgery may have an adverse effect on people willing to use this new technology. Despite this, the authors felt the lower early reoperation rate and patient benefits justified the use of the ioMRI and potentially lowered the costs in the long term.32
Other
Another problem that will become relevant to all caregivers as ioMRI suites proliferate is the looming shortage of helium, which is necessary for MRI as it used to cool the superconducting cable in order to essentially provide a higher strength and more stable magnetic field. There is growing concern that, although helium is one of the most plentiful elements in the universe, there is a looming shortage of usable helium on Earth. MRI machines typically lose a small amount of helium on a yearly basis as it boils off. Thus, it needs to be replaced over time. Some estimate the supply to be exhausted within 30–50 years.33 Researchers are currently attempting to develop MRI systems that cool the superconducting cable to appropriate temperatures without the use of helium, but these devices are still in development.34
Device Screening
Ferrous objects, including those brought by patients, visitors, contractors, etc., should be limited from entering Zone III, whenever practical. As part of the Zone III site restriction and equipment testing by MR technologists it is important to have ready access to a strong handheld magnet (≥1,000 Gauss). This will enable the site to test external, and even some superficial internal, devices or implants for the presence of grossly detectable ferromagnetic attractive forces. External devices or objects demonstrated to be ferromagnetic and MRI incompatible may still be brought into Zone III if, for example, they are deemed by MRI personnel to be necessary and appropriate for patient care. These devices should be accurately labelled and must be appropriately physically secured or restricted at all times during which they are in Zone III/IV. This is to ensure that they do not inadvertently come too close to the MR scanner and accidentally become exposed to static magnetic fields or gradients that might result in their becoming either hazardous projectiles or no longer accurately functional.28 The particular concern in the ioMRI suite is that MRI-unsafe items will be inadvertently left unaccounted for when the magnet deploys, putting both patients and staff at risk of projectile or thermal injuries. All staff working in ioMRI must be vigilant to prevent such an event from occurring.
Management of Emergencies
One of the major concerns to perioperative practitioners working in ioMRI environments is the safe management of crisis situations. This involves designing effective processes that allow controlled access for help in the event of a patient arrest or near arrest. There are many factors to consider when ensuring that an MRI facility is adequately and appropriately prepared to handle any of several types of emergencies that may occur. The American Society of Anesthesiologists (ASA) Taskforce on Anesthetic Care for Magnetic Resonance Imaging and ACR Guidance Document for Safe MR Practices cover some of these thoroughly and it is important that each institution is familiar with the recommendations.8,28 The process of resuscitating a patient during a diagnostic MRI can be chaotic enough, but when such a need arises in ioMRI, the process can be even more complicated. If the ioMRI suite is located in the main OR, there will be plenty of help available. The challenge is how to control access so that the people helping do not introduce hazards to the environment that may further injure the patient or other caregivers. Furthermore, the concepts of either a movable magnet or movable patient create a time period where personnel cannot get access to the room itself until the patient is no longer in Zone IV. For this reason, it is useful to always have at least two people in the room, even during scanning, in the event of an unexpected emergency. These people can initiate resuscitation until the patient is safely in Zone IV and other help can get safe access to the room.
If a patient has a medical emergency (e.g., cardiopulmonary arrest) in the ioMRI scanner, immediately remove the patient from Zone IV while initiating cardiopulmonary resuscitation, if indicated. Call for help and transport the patient to a previously designated safe area for resuscitation that is not in Zone IV if the magnet is stationary.8 In movable magnet/stationary patient systems, the magnet should be removed from the room (this can take several minutes) before additional help and a crash cart are allowed access in case further injury occur to the patient or staff. The presence of a ‘safety officer’ in the room can help to prevent inadvertent entry of well-meaning, but potentially hazardous, people and materials during a crisis situation. This previously designated person (nurse, MR tech, physician, etc.) can serve as a gatekeeper, preventing entry of help until the room is no longer part of Zone IV.
All MRI personnel should be aware of local policies and practices regarding the management of other emergencies such as fires, projectile emergencies and an MRI quench. It is strongly advised that all ioMRI facilities perform regular drills to rehearse and refine emergency response protocols to protect patients, MRI staff and responders. Simulation exercises may offer another opportunity to practice such rare, but serious scenarios.
New Directions
Although ioMRI was originally designed to facilitate improved localisation and resection of intra-cranial lesions, the uses of this technology have evolved. One new use of ioMRI involves thermal ablation of deep brain lesions using MRI-guided placement of laser catheters and real-time MRI guidance during the actual ablation.35,36 This technique, known as laser-induced thermal therapy (LITT), has been utilised to treat a variety of lesions including deep seizure foci and hypothalamic hamartomas. As the utility of diagnostic MRI continues to expand, it should be expected that a similar trajectory for ioMRI would continue.
Conclusions
Advances in technology such as ioMRI can offer improved patient outcomes while presenting anaesthesiologists with new challenges. Precise intra-cranial navigation is important to facilitate optimal outcomes in neurosurgical procedures. ioMRI represents the current state of the art with intra-cranial navigation. Outcome studies are lacking describing this technology. Future research is needed to demonstrate long-term outcome differences as well as define optimal patient selection and difference-making safety practices. An extensive knowledge of the unique considerations of delivering an anaesthetic in an MRI environment is vital to safe and effective patient care during procedures utilising ioMRI. The movable magnetic fields common to ioMRI represent a further challenge to anaesthesiologists. Although the challenges are significant, safe care and optimal outcomes are certainly possible with appropriate understanding of the matters unique to the ioMRI environment, good communication, a collaborative approach and proper procedural planning.