Alzheimer’s disease was first described in 1906, and yet we have still not fully elucidated the pathogenesis of the condition. It is known that the disease is characterized by the polymerization of amyloid β-peptide (Aβ), leading to the formation of plaques.1,2 The cascade of events initiated by Aβ polymerization eventually leads to progressive neurodegeneration and brain dysfunction. However, we have not yet established how and exactly where in the cell Aβ is generated from amyloid precursor protein (APP) and where the polymerization takes place. One technical challenge is the fact that most subcellular compartments cannot be spatially resolved by traditional confocal light microscopy. New technological advances such as super-resolution microscopy have enabled us to visualize the location of Aβ and γ-secretase, the enzyme complex that liberates Aâ from its precursor APP, in neurons.
In an expert interview, Lars Tjernberg and Sophia Schedin Weiss discuss their microscopy studies that are elucidating the molecular pathways that underline Alzheimer’s disease.3–5
Q. What is your view on the pathogenesis of Alzheimer’s disease?
LT: The most important factor in Alzheimer’s disease is a small piece of protein – the Aβ – that comes in different lengths. One peptide with 42 amino acids has been found to be toxic in laboratory studies with cell lines and in animal studies.6,7 Deposits of this peptide as amyloid plaques were first found in the human brain over 100 years ago. Now we have a clear understanding on how Aβ is produced. The final cleavage reaction that produces Aβ from its APP is mediated by γ-secretase. A number of clinical trials have aimed to stop Aâ production by inhibiting this γ-secretase enzyme complex.8,9 Unfortunately, γ-secretase has around 100 different substrates and therefore we need to be more specific in inhibiting Aâ production.
Q. Why did you initiate these studies?
LT: We wanted to find out exactly where in the cell Aβ is produced from its precursor. To achieve this, we wanted to use neurons rather than cell lines that are used in many other studies. Neurons were obtained from mice and cultured in vitro, allowing them to form synapses. When examining these cells under the microscope we discovered that γ-secretase is concentrated at synapses, both at the pre-synaptic and post-synaptic sides.3 It is located in small, spherical structures called vesicles on both sides and at the post-synaptic side it is also located at the cell membrane. When we repeated these studies to locate Aβ, we found that it was only located at the pre-synaptic side in similar structures, in many cases containing a protein called synaptophysin, which means that these are vesicles involved in synaptic transmission.
Q. How can you obtain such impressive resolution?
SSW: Light microscopes are commonly used to look at cells and tissues. Fluorescence microscopes are a type of light microscope that take advantage of the fact that certain molecules, known as fluorophores, excited by light of a certain wavelength, can emit light of another wavelength. By using fluorophores coupled to compounds that bind specifically to certain molecules, we can visualize specific components in cells. Since light propagates as waves, the light hitting the sample diffracts. Thus, even using an objective lens, we cannot focus the light to more than half the wavelength, which is usually around 200–350 nm.
Particles smaller than this cannot be resolved, however, scientists have discovered that fluorophores, which emit light, can be switched into dark states. This fact has been taken advantage of to overcome resolution limitations caused by the diffraction. One way to achieve this is to turn molecules dark by stimulated emission.10 This can be achieved in specialized laser scanning microscopes by overlaying the excitation laser light with a depletion laser light in a donut-shaped pattern at the edges of the fluorescent spot. This switches off the fluorescence at the edges of the spot, minimizing the size of the spot, resulting in improved resolution, as if we could paint using a thinner brush.
Another principle used to increase resolution is known as single molecule localization microscopy.11,12 With this technique, the fact that certain fluorophores under certain circumstances can switch between fluorescent and dark states is used, which results in stochastic blinking of the sample when illuminated with high power laser light. By using very rapid cameras it is possible to produce a series of images where only a few fluorophores are turned on in each image. These can be resolved from each other and the exact center of each fluorescent spot can be determined. By adding together the data from such a series of images, a super-resolved image can be reconstructed.
These two examples represent ways to achieve superior resolution in different types of fluorescence microscopes. We can achieve at least tenfold improved resolution compared with confocal microscopes. This makes it possible for us to visualize small structures in the cells where Alzheimer’s disease pathology takes place, such as the small vesicles at the synapses.
LT: To give you some idea of the dimensions we are looking at, the synaptic vesicles are around 40 nm in diameter and the synaptic cleft, i.e., the distance between the pre- and post-synapse, is around 20 nm. With the traditional confocal microscope we have a resolution of around 200 nm. With our system, we have a resolution of 10–30 nm, so this technique is perfect to resolve the structures that we are studying.
SSW: Although many previous studies have been performed on Aβ and γ-secretase, we have not seen any other publication where they have used these super-resolution microscopy techniques to image γ-secretase and Aβ in neurons.
LT: I think we will see many more similar studies since these microscopes are becoming available to more and more scientists and people realize how much they can improve their studies by using these techniques.
Q. What are the major findings of your investigations?
LT: The major findings were that γ-secretase is located both pre-synaptically and post-synaptically,3 whereas Aβ is only located at the pre-synapse,4 potentially in synaptic vesicles. When we see these well-defined structures containing γ-secretase and Aβ, we realize that they must be there for a reason and we should be very careful when we try to limit Aβ production, the most popular treatment approach, in order to treat AD. Since γ-secretase has so many substrates, treatment needs to be very specific. One possibility would be to target γ-secretase at the pre-synapse and leave the other side untouched.
Q. How could your results help us treat Alzheimer’s disease?
LT: These results may be a starting point for thinking how to reach this particular pool of Aβ at the pre-synapse and also that we should not reduce Aβ production too much because it may have a physiological function. Our studies suggest that we can be more specific in our treatment approaches.
SSW: Since we identified different types of vesicles that contain Aβ in the neurons, one of our aims is to identify the nature of these vesicles.
LT: We think that there are other types of vesicles and we want to define the differences between them. We also want to try some pre-commercial microscopes that have super-resolution in all three dimensions. This will allow us to make three-dimensional, super-resolved images and thus characterize the vesicles even better.
Q. Where will you go from here?
SSW: We have observed Aβ42 in different types of vesicles in the neurons. Some of these are synaptic vesicles, but we have also seen other types of vesicles that contain Aβ42. We aim to identify the nature of these vesicles using more super-resolution studies. We also aim to set up live cell imaging experiments so we will be able to follow exactly where in the cell Aβ is formed. This will allow us to view how Aβ is transported inside the cells and where it starts to aggregate, as well as how it can be transferred from cell to cell. All these studies will be performed in neurons with super-resolution.
LT: We also have ways of looking at Aβ when it polymerizes. Aβ is produced as a monomer and initially it is not toxic. When it starts to polymerize, it forms toxic oligomers. Combining the super-resolution techniques that we use with a novel Aβ labeling technique that we develop we will be able to follow the polymerization process in living neurons and elucidate exactly where it takes place. ⬛
Figure 1: Super-resolved image of Aβ42 vesicles at the synapse of hippocampal neurons
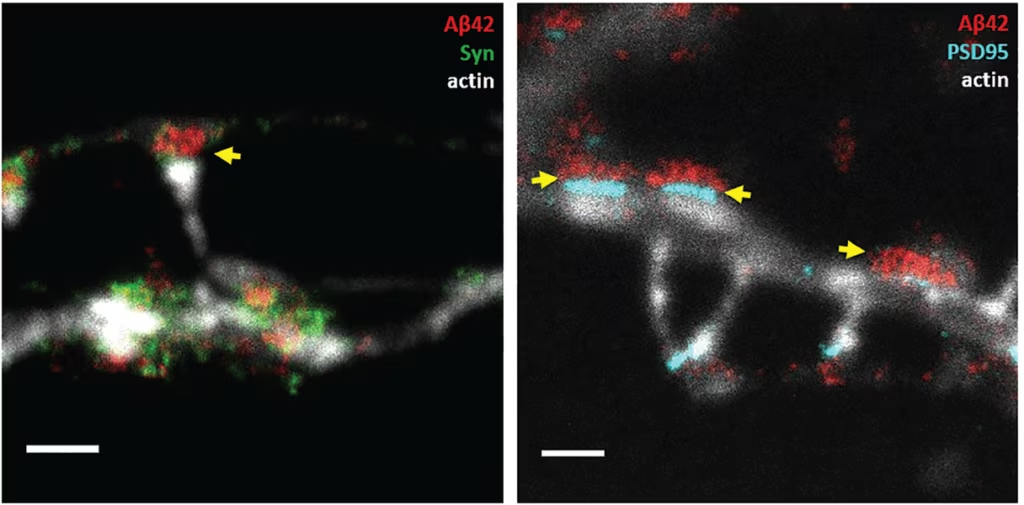
Stimulated Emission Depletion (STED) microscopy was used to image synapses of immuno-labeled primary hippocampal neurons. Left: Aβ42 (red) and the pre-synaptic marker synaptophysin (syp, green). Right: Aβ42 (red) and the post-synaptic density (cyan). Neuronal structure is shown by actin in a confocal channel (light grey) in both images. Scale bars,
1 µm. Yellow arrows point at Aβ42 vesicles in the pre-synapse. In the right image, the distance between the post-synapse and the pre-synaptic Aβ42 vesicles is around 25 nm.
Aβ = amyloidβ-peptide.














