Affecting over 70 million patients worldwide, epilepsy is a chronic neurological disorder characterized by intermittent bursts of hyper-synchronous neuronal discharges.1 The manifestations are variable but reflective of the unique milieu and biology of epileptogenic foci.2 Pharmacological treatment with antiepileptic drugs (AEDs) is supportive only and, for up to 30% of patients, is inadequate in preventing seizures.3 While surgical resection can be curative in refractory cases of temporal lobe epilepsy (TLE), it entails risk of cognitive impairment.4 With the success of stem cell therapy in catalysing regeneration in spinal cord injury and dopaminergic neuronal replacement in Parkinson’s disease, interest was spurred in exploring its applicability to other central nervous system pathologies that could potentially benefit from stem cell-induced plasticity and restoration.5–7
Recently, the molecular mechanisms that culminate in baseline neuronal hyper-excitability (epileptogenesis) and, eventually, seizures (ictogenesis) have been active areas of investigation.2 Animal models of epilepsy and patient samples have been vital in unveiling these mechanisms, which range from dysregulated neuronal ionic flow to imbalances between inhibitory gamma-aminobutyric acid (GABA) and excitatory glutamate neurotransmitter levels.2 While the involvement of neuronal networks has traditionally been emphasized in the literature on epilepsy, there is a growing interest in the role of extraneuronal cells. Through the secretion of neurotrophic peptides, such as glial cell-line-derived neurotrophic factor (GDNF), astrocytes are known to exert an anticonvulsant effect. The loss of GDNF expression, indeed, has been demonstrated in a rat model of epilepsy.8 Astrocytes also uniquely serve as the site and effectors for the metabolism of adenosine, an endogenous anti-epileptic molecule, which activates central A1 receptors, terminates seizures and mediates post-ictal refractoriness.9 Befitting their duties as first responders, cells of the innate immune system are especially sensitive to stress accumulated through recurrent seizures. Repeated seizures lead to a build-up of oxidative stress within neuronal mitochondria, leading to neuronal death and activation of toll-like receptors of the innate immune system. This sets off a positive feedback loop of glial immune activation, neuronal loss and seizure progression.10
The role of endogenous stem cells in epilepsy
In addition to glial and immune cells, the resident niche of stem cells also modulates the neuroinflammatory milieu in the epileptic brain. These pockets of regenerative cells, like all stem cells, are characterized by their ability to self-renew, replicate and differentiate into multiple different cell lineages (Figure 1). Early in the epilepsy course, there is a transient increase in aberrant neurogenesis in the hippocampus, where specialized collections of resident neural stem cells (rNSCs) are typically found. Sierra et al. showed that following seizures, rNSCs divide and differentiate into astrocytes, leading to astrogliosis of the hippocampus.11 Reactive astrocytes harbour impaired glutamate transporters, which are conducive to epileptogenesis. Parent et al. found that seizures maladaptively stimulate chainlike configuration and movement of dentate granule cell (DGC) progenitors from the subgranular zone of the hippocampus into the dentate gyrus hilus and molecular layer.12 The resultant hilar ectopic DGCs exhibit abnormal network integration and hyper-excitability, further contributing to the epilepsy phenotype.
Figure 1: Cellular pathways and delivery routes
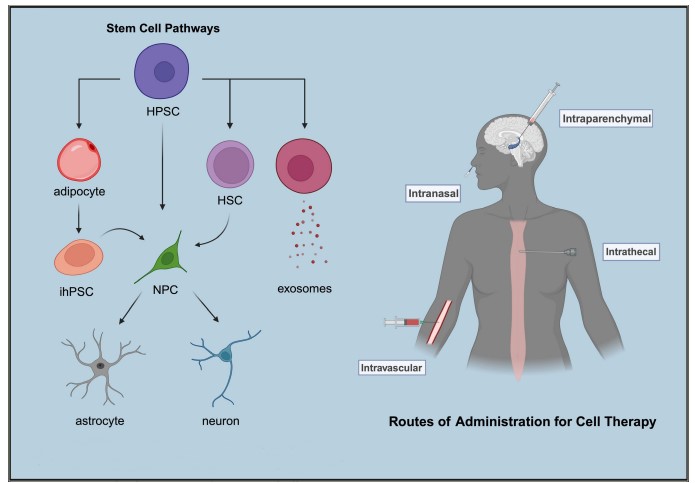
Left illustrates the pathways through which stem cells can naturally evolve or be induced into specialized cell types during the differentiation process. Right demonstrates the various routes of administration that can be employed to deliver cell therapy. Created with BioRender.com.
HPSC = human pluripotent stem cell; HSC = haematopoietic stem cell; ihPSC = induced pluripotent stem cell; NPC = neural progenitor cell.
Stem cell-derived therapeutic strategies
Combined evidence from in vitro and in vivo experiments has shown that stem cell-derived neural progenitors, upon transplantation, can exert therapeutic effects via two primary mechanisms: by differentiating to allow for region-specific functional engraftment into adult neuronal networks or by staying undifferentiated to assert a bystander effect.13,14 Carpentino et al. showed that seizures induce stem cell differentiation into Prox1+ neurons and GFAP+ astrocytes and their integration into dentate gyrus and CA3 regions while suppressing grafted stem cells from undergoing tumorigenesis.13 Huang et al. demonstrated that transplanted human umbilical mesenchymal stem cells (MSCs) can survive within host hippocampi, retain an undifferentiated state and confer neuroprotective changes by expressing several trophic factors.14 These findings have particularly prompted researchers to investigate if the propensity of stem cells and their progeny to modulate their environment also applies to their secretions. Kodali et al. showed that intranasally administered extracellular vesicles procured from human bone marrow MSCs can efficiently incorporate into neurons and microglia in the injured forebrain of status epilepticus (SE) rodent models.15 Analyses of factors secreted by stem cells (secretome) reveal copious anti-inflammatory cytokines (glucocorticoid-induced TNFR-related [GITR] and fibroblast growth factor 6 [FGF–6]) and neural growth factors (FGF-2 and brain-derived neurotrophic factor [BDNF]). This cocktail of biomolecules has the potential to be used as a substitute to direct stem cell implantation to repair the blood–brain barrier, recoup neurotrophic factor levels and dampen inflammatory processes implicated in epileptogenesis.16 With advances in cell engineering, stem cells can now also be genetically modified using viral vectors to circumvent myriad defects, such as adenosine receptor imbalances or calcium channel abnormalities. Huicong et al. use human MSCs transduced with cassettes of microRNA directed against adenosine kinase, an enzyme responsible for the adenosine breakdown, to restore adenosine balance in the rat hippocampus.9
As the loss of GABAergic interneurons in the hippocampus is strongly associated with TLE, multiple animal studies have used an array of sources (umbilical, foetal, or adult stem cells) and techniques (infusion or implantation) to supply hippocampi with progenitors that can give rise to phenotypic inhibitory inter-neurons.17 Hippocampal injury caused by SE has been consistently shown to lead to chronic epilepsy in animal models of TLE. While AEDs can terminate SE, they have not been proven to inhibit epileptogenesis and subsequent TLE. By transplanting embryonic stem cell-derived GABAergic progenitors into the hippocampi of rats perturbed with SE, Upadhya et al. were able to halt the progression into the chronic epileptic phase.18
In rodents with established chronic epilepsy, Waldau et al. cultured neural stem cells obtained from medial ganglionic eminence (MGE) and surgically grafted them into the dentate gyrus, generating a sustainable population of GDNF-secreting astrocytes and GABAergic interneurons.8 The graft led to a reduction in the duration and severity of spontaneous recurrent seizures in rats with chronic epilepsy. MGE cells have also been used in absence seizure models. Using “Stargazer mice” as absence seizure models, Hammad et al. used an immunoreactive marker of neuronal activity, c-fos, to identify the site of ictogenesis within the primary visual cortex (V1) and subsequently transplanted embryonic MGE stem cells into V1, causing a dramatic decline in absence seizure events.19 Furthermore, Hunt et al. found that focal injections of MGE cells into the hippocampi of epileptic mice restored spatial learning while decreasing hyperactivity and aggressiveness.20 This is encouraging, given that the cognitive and behavioural comorbidities that accompany epilepsy are known to significantly affect the quality of life of many patients, but are often unresponsive to medications.
Safety and efficacy
The safety and efficacy of stem cell transplantations in humans have been investigated in several clinical studies. Milczarek et al. intrathecally dispensed bone marrow-derived autologous CD271+ MSCs to paediatric patients with drug-resistant epilepsy, resulting in gradual yet marked clinical improvement with each transplantation and no major side effects over the 2-year follow-up.21 DaCosta et al. treated a cohort of 20 patients with mesial TLE by selective intra-arterial autologous infusion of mononuclear cells isolated from bone marrow.22 Up to 40% of patients were seizure-free at the 6-month follow-up, with no reported significant adverse reactions. Encouraged by the success of cell therapy in many autoimmune diseases, Szczepanik et al. assessed the utility of autologous intrathecal infusion of MSC-like cells isolated from liposuction-harvested adipose tissues in patients with drug-resistant epilepsy of either possible or proven autoimmune origins.23 Responses ranged from complete seizure freedom to mild reduction in frequency.
Active clinical trials
With the current advances in techniques, a large amount of low-immunogenic pluripotent stem cells can be induced, which are ethically obtained from adult somatic cells (e.g. fibroblasts and adipocytes) and stereotactically transplanted into brain areas of interest.24 Leveraging these findings, numerous multicentre clinical trials (Table 1) are actively recruiting patients with treatment-resistant epilepsy for cell-based therapies.25,26
Table 1: Current clinical trials investigating patients with treatment-resistant epilepsy for cell-based therapies
| Clinical trials | Location(s) |
| First-In-Human Study of NRTX-1001 Neural Cell Therapy in Drug-Resistant Unilateral Mesial Temporal Lobe Epilepsy25 ClinicalTrials.gov identifier: NCT05135091 Condition: Mesial temporal lobe epilepsy with hippocampal sclerosis Drug: GABA-secreting inhibitory interneurons (NRTX-1001) Source: Allogeneic human embryonic stem cells Start date June 2022 — Estimated completion date May 2026
| CA, IL, IA, MA, NY, NC, OR, PA, TX, UT, WI |
| Induced Pluripotent Stem Cell Derived Exosomes Nasal Drops for the Treatment of Refractory Focal Epilepsy26 ClinicalTrials.gov identifier: NCT05886205 Condition: Refractory focal epilepsy Drug: Exosomes (GD-iEXo-002) Source: Induced pluripotent stem cells Start date June 2023 — Estimated completion date November 2025
| Beijing |
GABA = gamma-aminobutyric acid.
Conclusion
Stem cell-based therapeutic strategies hold significant promise for the future in the treatment of medically refractory epilepsy. As we gain insights from early clinical trials, we will continue to optimize production and delivery mechanisms to help achieve safe, affordable and scalable methods of administering these potentially paradigm-shifting cellular therapies.














