Migraine is one of the most prevalent and disabling diseases worldwide, affecting more than one billion people in 2016.1 It is characterized by recurrent headache attacks with nausea/vomiting, photophobia and phonophobia.2 Currently, the first line of acute treatment of migraine is pharmacological; however, pharmacological approaches may be ineffective, poorly tolerated, and need to be limited in use in order to avoid medication-overuse headache and migraine chronification.3–5 Moreover, pharmacological treatment may not suit all patients, especially sensitive populations, such as pregnant women, children and adolescents, or patients with pre-existing medical conditions.6 Thus, there is a significant unmet need for alternative, non-pharmacological acute migraine therapies that are both effective and well tolerated.4 Non-invasive neuromodulation represents an emerging alternative for the acute treatment of migraine,7,8 and can even be cost-effective in certain instances.9 However, the efficacy of most neuromodulation devices has seemed inferior to that reported for migraine-specific pharmacological treatments.10–13 The aim of this paper is to provide a subjective review of peer-reviewed, evidence-based literature on remote electrical neuromodulation (REN) and its applications.
Remote electrical neuromodulation: Mechanism of action
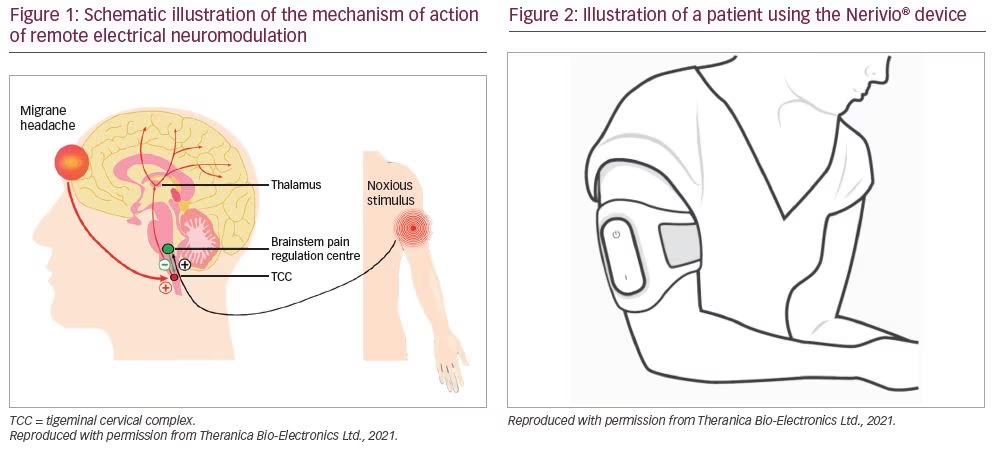
Remote electrical neuromodulation is a novel neuromodulation treatment in which upper arm peripheral nerves are stimulated to induce conditioned pain modulation.14 This is an endogenous analgesia mechanism in which conditioning stimulation inhibits pain in remote body regions (e.g. the head) (Figure 1). Nerivio® (Theranica Bio-Electronics Ltd., Netanya, Israel) is a wireless, wearable, battery-operated REN stimulation unit controlled by a smartphone software application.
During migraine headaches, nociceptive (pain) information descends from the affected areas in the head to the trigeminal cervical complex (TCC).15 Peripheral REN on the upper arm (Figure 2) activates nociceptive sensory fibres, predominantly A-delta fibres in the epidermis, above their depolarization thresholds but below the perceived pain threshold (in other words, activating sensory receptors without causing pain). This information ascends to the brainstem pain regulation centre (involving the periaqueductal grey, rostral ventromedial medulla and subnucleus reticularis dorsalis). The processing of this information in the brainstem results in the release of serotonin and noradrenaline. These neuromodulators create a global inhibitory effect, including the inhibition of the painful migraine messages in the TCC, leading to relief of migraine pain and other symptoms.16,17
Clinical evidence supporting the safety and efficacy of the remote electrical neuromodulation device
The REN device is US Food and Drug Administration (FDA) indicated for acute treatment of migraine with or without aura in patients 12 years of age or older. Following physician prescription, treatment is self-administered within 1 hour of onset of migraine headache or aura. The device is applied to either lateral upper arm, between the bellies of the lateral deltoid and the triceps. Users control stimulation intensity via a wirelessly connected smartphone application, bringing stimulation to an individualized intensity that should be well perceptible (strong enough to activate A-delta fibres to induce the conditioned pain modulation) yet low enough to maintain the overall sensory experience below the perceptual pain threshold (i.e. not painful). The app further includes a personal migraine diary, which can be used to track migraine symptoms and treatments.
The safety and efficacy of the REN device has been established in a series of five independent clinical trials involving more than 500 participants with migraine disease.16,18–21 These comprehensive studies comprised a diverse group of participants, with both episodic and chronic migraine profiles included, as well as a wide age range involving people 12 years old and above. These results were further repeated in a large, post-marketing surveillance study with 4,725 patients with migraine.22 Since the publication of the latter real-world study, and as of mid-February 2021, many additional patients have used REN to treat acute migraines (personal communication, Theranica Bio-Electronics Ltd., 2021). This totals more than 11,000 patients using the device for more than 100,000 treatments in the USA, in addition to over 200 patients completing more than 1,500 treatments in Israel (following Israeli regulatory approval in March 2020).
Summary of safety
In all studies (clinical trials and real-world evidence), the incidence of device-related adverse events was low (ranging between 0.5 and 3.6% of patients, and between 0.34 and 4.8% of treatments). These included a warm sensation; pain/soreness in the arm, hand, shoulders and/or neck; local redness of the skin; local itching or tingling; temporary arm/hand numbness; or muscle spasm. All adverse events were categorized as ‘not serious’ (mild), resolved within 24–48 hours of treatment,16 and none of the participants withdrew from the studies due to adverse events.16,19,20,22,23
Summary of efficacy
A recent comprehensive systematic review and meta-analysis of 38 randomized controlled trials focusing on neurostimulation migraine treatments identified REN as the only effective neurostimulation treatment for acute migraine. Data were insufficient to draw conclusions for any other technique, as other neuromodulation techniques were tested in single studies.6
Aggregated data from REN studies provide cumulative evidence for acute treatment efficacy at 2 hours post-treatment, as well as sustained effects 24 hours post-treatment. Cumulatively, over REN studies,16,18,19 59.3–71.8% and 20.9–37.4% of patients reported pain relief and pain freedom, respectively, at 2 hours post-treatment with a single REN session, independent of pain severity before treatment.16,19 Benefits were maintained over subsequent attacks, with 51.7–73.7% and 20.0–44.4% of patients experiencing consistent pain relief and pain freedom, respectively, in at least half of REN-treated acute attacks.16,18,19,21,23 Long-term stability was evident in terms of consistent efficacy of pain relief and pain freedom over multiple consecutive attacks.20 Pain relief and pain freedom were sustained for 24 hours in 46.6–90.9% and 46.9–90.9% of patients, respectively, across all patient populations, clinical trials and real-world evidence.18,19,22
The positive effects from REN treatment are not limited to headache. REN also leads to the disappearance of at least one associated migraine symptom, such as nausea and/or vomiting, photophobia, phonophobia, or the most bothersome symptom (MBS) of these associated symptoms, in 45.9–65.8% of patients. Even more importantly, improvement in functional ability was seen in 45.7–69.7% of the participants.18,19,20,22
Sham-controlled trials showed that the efficacy of REN was significantly superior to a sham stimulation differing in pulse width and frequency (100–120 Hz for 50–200 μs versus 0.083–0.1 Hz for 45 μs).16,21 For patients treated by either REN or pharmacological treatments, the efficacy of REN is not inferior to standard of care pharmacological treatments in relieving or eliminating pain at 2 hours post-treatment, and is superior to standard care (i.e. pharmacological medications or no treatment) in achieving pain relief at 2 hours post treatment (reported by 66.7% of participants when they used REN versus 52.5% of the same participants under their usual care, p<0.05).24
Safety and efficacy in adults with episodic migraine
A series of studies evaluated the safety and efficacy of REN in adults with episodic migraines. The first REN study conducted provided initial evidence that non-painful remote electrical stimulation on the upper arm can significantly reduce acute migraine headache in patients with episodic migraine, especially when applied early in an attack.21 The study was a prospective, double-blind, randomized, crossover, sham-controlled trial. Adults (n=86; aged 18–75 years) with migraine with or without aura who met the International Classification of Headache Disorders (ICHD-3 beta) with 2–8 attacks per month participated. Seventy-one participants (299 treatments) successfully treated at least one migraine attack with REN, while refraining from medication use for 2 hours from stimulation onset. No adverse events were reported.21
This first study explored a few stimulation-related parameters to determine optimal treatment: (1) Several stimulus-response relationships were tested, given at a random sequence per participant. A 2 x 200 μs pulse width generated the optimal efficacy, while shorter-pulse treatments (2 x 50, 2 x 100, or 2 x 150 μs) were less efficacious. All active stimulations were 100–120 Hz, and the placebo stimulation was 0.1 Hz with 45 μs pulses. Note that 200 μs refers here to half (positive or negative) a cycle; later studies refer to the full stimulus cycle (i.e. 400 μs pulse width). (2) Pain reduction was highest when applied within the first 20 minutes of attack onset, while efficacy dropped for treatments that began later than 60 minutes from migraine onset. (3) Treatment duration of 20 minutes was not effective for some participants; however, two sequential treatments (40 minutes) led to higher rates of relief. Thus, this study set the ground for optimal treatment parameters used in the studies that followed: 45 minutes’ stimulation of proprietary symmetrical, biphasic, square electrical signal with a modulated frequency of 100–120 Hz, pulse width of 400 μs, and output current up to 40 mA (adjusted by the patient), applied as soon as possible and always within 1 hour of migraine onset.
The following pivotal study,16 classified as high quality,6 serves as one of the largest randomized, double-blind, sham-controlled device studies for acute migraine to date. Participants (n=296; aged 18–75 years) meeting the ICHD-3 beta criteria for migraine with or without aura with 2–8 migraine headaches per month but fewer than 12 headache days per month, were enrolled in the multicentre trial. Following a 2–4-week baseline (run-in) phase, during which attacks were treated according to usual care and tracked in an electronic diary application, eligible participants (n=252) were randomized in a 1:1 ratio to active (optimal REN protocol as mentioned above) or sham stimulation (pulse frequency of ~0.083 Hz and width of 40–550 μs) for 4–6 weeks’ treatment at home. Efficacy analysis was based on treated attacks preceded by at least 48 headache-free hours, to avoid treating recurrent headaches; attacks in which rescue medications were not used within 2 hours post-treatment, to avoid treatment interactions; and attacks with diary entries at stimulation onset and 2 hours post-treatment.
The statistically significant and clinically important benefits of REN compared with sham were demonstrated 2 hours after REN treatment of acute migraine attacks in regards to pain relief (66.7% versus 38.8%, p<0.0001; therapeutic gain of 27.9%), pain freedom (37.4% versus 18.4%, p=0.003), relief of the MBS (46.3% versus 22.2%, p=0.0008) and the combination of pain relief and MBS relief (40.0% versus 15.2%, p=0.0004). The clinical benefits of REN sustained 48 hours post-treatment were maintained in subsequent attacks and were independent of baseline pain levels.16 The difference between the active and sham groups was not affected by the participants’ perceived assignment, as tested upon treatment completion. REN also provided a favourable safety profile demonstrating a low incidence of device-related adverse events, rates of which were similar between the two groups (4.8% versus 2.4%,
p =0.499). Results from this pivotal study were further discussed in two review papers.7,24
A follow-up phase of this study, and a post hoc analysis comparing its treatment phase with the baseline (run-in) phase, further evaluated REN efficacy relative to standard care pharmacological treatments.23,25 The post hoc analysis compared REN efficacy from the randomization phase with usual care efficacy (including either migraine-specific and non-specific pharmacological treatments or no pharmacological treatment) from the study baseline phase prior to randomization (n=99 participants available for this analysis).25 Results showed that, in at least some participants, REN treatment was even more efficacious than usual treatment in terms of pain relief at 2 hours post-treatment, while efficacy did not differ between REN and usual care in regards to pain-free status at 2 hours. Within-subject analysis showed REN treatment was as effective as pharmacological treatments (including any type of acute medication) in achieving either pain relief or pain freedom at 2 hours post-treatment, both in a single attack and across two attacks. Importantly, the efficacy of REN does not depend on whether or not patients are taking preventive medications.
In an open-label extension of the pivotal trial, participants had the opportunity to incorporate REN into their usual care for 8 weeks following the randomization phase. Within-subject analyses (n=117) compared medication use patterns between usual care during the baseline phase (as in the previous study, i.e. usual care with or without medications) and the open-label extension phase (i.e. when REN was available in addition to usual care). During the open-label extension, when REN was available, many more participants treated their attacks only with REN, avoiding medications in all their attacks, compared with a smaller number of patients who avoided any medications during baseline phase (89.7% versus 15.4% participants, p<0.0001), independent of group assignment during the randomization phase. Similar to the exploratory post hoc study, rates of pain relief and pain freedom in at least half of the treatments at 2 hours post-treatment were comparable between the two phases of the study.7,23
Taken together, these clinical trials provide peer-reviewed evidence for REN as a device that provides clinically meaningful benefit in treating acute migraine attacks in people with episodic migraines compared with placebo sham stimulation, with a low incidence of adverse events and good tolerability. The potential of REN to reduce acute medication use while maintaining similar efficacy to usual care, i.e. without hindering the relief of migraine pain, is demonstrated.
Safety and efficacy in adults with chronic migraine
The studies reported so far focused on episodic migraine. Slow increase in headache frequency over months and years might lead to chronification, resulting in chronic migraine with 15 or more headaches a month. The risk of developing chronic migraine can depend on the frequency and type of acute medications used.26 There is, therefore, great need for non-pharmacological acute treatment to avoid migraine chronification, and to reduce the frequency of migraine attacks and migraine-related disability while avoiding the overuse of migraine acute medications in chronic migraine. Patients have identified the consistency of treatment over multiple migraine attacks as a desirable attribute of acute migraine therapies.27
The safety and efficacy of REN for patients with chronic migraine was assessed in two prospective, open-label, single-arm studies. Adults (n=91 and n=42; aged 18–75 years) with chronic migraine participated in 4-week trials with the REN device.18,20 Analyses specifically focused on intra-individual consistency of pain response across multiple attacks (defined, as in previous studies, by efficacy in at least half of the treated attacks). Given the higher frequency of attacks in patients with chronic migraine, and to provide additional meaningful insights, long-term treatment responses were analysed across the first five consecutive evaluable treatments per participant.
Similar findings were reported in both studies. Participants experienced pain relief and pain freedom in the first qualifying treatment (59.3% and 20.9%, respectively).18 Effects were consistent in the other trial, reported in at least half of their treated attacks at 2 hours post-treatment (73.7% and 26.3%, respectively).20 Data reveal long-term consistent response rates: 50.0–63.3% and 23.3–27.0% pain relief and pain-free responses, respectively, at 2 hours post-treatment over the first five consecutive evaluable treatments.20 Improvement in functional ability was reported in both studies at 2 hours post-treatment (45.7%20 and 59.3%18). Single device-related adverse events were reported in each study (1.8%20 and 1.0%18 of participants). Participants avoided medications in 89.5% of the 210 qualifying treatments, and 57.9% of the participants did not use medications within 2 hours of treatment onset in any of their treatments during the study.20
Demonstrating consistency of a treatment is clinically important, especially in a chronic migraine population who experience numerous attacks per month, thus increasing confidence in treatment efficacy while increasing adherence and reducing migraine-related disability and medication overuse.28
Safety and efficacy in adolescents
Migraine prevalence increases with age, particularly in adolescents. A novel study evaluated the safety and efficacy of REN treatment in adolescents (n=45, aged 12–17 years) with either episodic migraine or chronic migraine meeting ICHD-3 criteria for migraine with or without aura, who participated in an open-label, single-arm, multicentre study.19
A single (non-serious) device-related adverse event (2%) was reported, in which a temporary feeling of pain in the arm was felt and was resolved after the treatment without requiring intervention. Of 39 participants completing at least one REN treatment (159 qualifying migraine headaches), pain relief and pain freedom at 2 hours were achieved by 71% and 35% participants, respectively; both sustained for 24 hours in 90% of the participants. Improvements were consistent over treatments, with 66% and 33% of participants experiencing pain relief and pain freedom in at least half of the treated attacks, respectively. Disappearance of at least one of the associated symptoms and improvement in functional ability were experienced at 2 hours post-treatment (66% and 69% participants, respectively). Compliance rates were extremely high, as only two participants used rescue medications within 2 hours post-treatment, and only a single participant began treatment over an hour from attack onset.
This study allows for the extension of results in adults, demonstrating the safety, tolerability and efficacy of REN for acute migraine treatment in adolescents suffering from either episodic or chronic migraines, showing consistent and sustained clinical benefits.
Real-world evidence
Randomized controlled trials are the gold standard of clinical research, designed to eliminate bias and ensure reliable evaluations. Yet results obtained in a controlled environment may not reflect how a therapy would be used in everyday practice, in a variety of environments and in more diverse patient populations who are not screened, monitored or consistently provided with training and guidance.
To evaluate the safety and effectiveness of REN for acute migraine treatment in a real-world setting, a prospective, post-marketing surveillance trial in clinical practice was conducted during the first 6 months that Nerivio® was available in the USA, following FDA approval.22 The study population included all patients (aged 12 years and older) who used the device at least once (n=4,725 patients, total of 25,984 treatments), creating one of the largest real-world data sets among patients with migraine. Patients were stratified based on the type of visit and prescription provider: in-person visits with headache specialists (HS group) or virtual visits with non-headache specialists (non-HS group; note that telemedicine by headache specialists was not available in the USA at the time of this data collection). This distinction is important because those seeking in-person headache specialist care mostly represent the chronic migraine population, having a high frequency of attacks with higher proportion of severe baseline pain and higher rates of moderate to severe migraine-related functional disability, while those in the non-HS group involved patients experiencing episodic migraine.
Patients had the opportunity, but were not obliged, to record their symptoms in real time in the REN application diary, and thus, efficacy analyses included patients who had performed at least one evaluable treatment in which pain levels were provided at treatment onset and at 2 hours post-treatment, and no medication was taken (n=1,384 patients, total of 2,953 treatments; of these, 1,339 patients and 2,875 treatments were included in the HS group). Efficacy focused on intra-individual consistency of response across multiple attacks, defined as response in at least half of treated attacks per parameter, given the clinical importance of consistent efficacy and tolerability, especially in a real-world setting.22
Most patients experienced pain relief (58.9% and 74.2% of the patients in the HS and non-HS groups, respectively) and many experienced pain freedom (20.0% and 35.6% of the patients in the HS and non-HS groups, respectively) in at least half of their treated attacks at 2 hours post-treatment. The effects of REN on associated symptoms and improvement in function were also consistent in both groups. Pain responses were sustained at 24 hours in approximately 46% patients, demonstrating long-lasting efficacy and a low rate of migraine recurrence. The incidence of device-related adverse events was very low (0.5%), and all adverse events were resolved within 24 hours and categorized as not serious.22 Updated real-world data from mid-February 2021 (personal communication, reported by Theranica Bio-Electronics Ltd., 2021) revealed that patients avoided taking any medications within 2 hours post-REN treatment in 51.5% of the 14,998 treatments in which medication use was reported.
Overall, the clinical benefits of REN in the real-world setting were similar to those reported in randomized controlled trials and open-label extension, and, as expected, higher in the non-HS group, representing the broad migraine population. The current data also support the willingness of patients to adopt a drug-free treatment, suggesting that the device may reduce reliance on medication, and consequently may reduce the risk of developing medication-overuse headache, a common concern for patients suffering from chronic migraines.
Future studies
The current literature and clinical trials involving REN have several limitations. First, consistent efficacy and tolerability over multiple migraine attacks have been shown in 4–8 weeks of clinical trials, as well as long-term over five consecutive treatments in chronic migraine patients. It would be beneficial to further evaluate the consistency of efficacy over even longer terms (months, years) via real-world evidence. It is also especially important to assess the effects of REN on medication-overuse headache. Consideration should be given to the importance of investigating whether REN can be used to treat patients who have already developed medication-overuse headache, by assisting their withdrawal or wean-off of the offending medications, while shifting to REN treatment. Most of the studies reviewed here evaluated the efficacy of REN in groups of patients (i.e. looking at the portion of participants from the entire tested pool who reported a specific outcome). Within-subject analyses have the potential to further refine and personalize treatment. Advanced analytics on real-world data, looking at the frequency of migraine attacks, efficacy of treatments and combined treatments (REN, medications, other treatments), and additional diary input can be used to optimize treatment, health economics and quality of life for individual patients.
Conclusions
REN represents an innovative, safe, effective, well-tolerated, non-invasive, non-pharmacological alternative for the acute treatment of migraine in individuals aged 12 years or older. It may be especially important for sub-populations who cannot use pharmacological therapies because they do not respond to them, have contraindications to them, are unable to swallow medications, have more migraine attacks than the recommended dose of medications allows for, cannot tolerate the side effects of drugs, or they simply prefer a drug-free lifestyle.