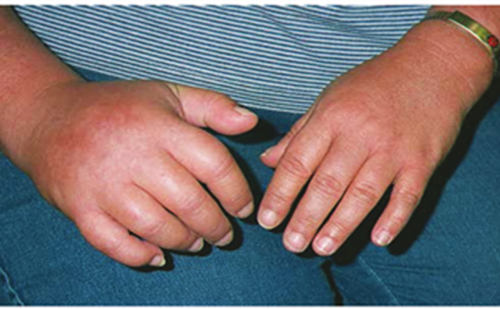
Complex regional pain syndrome (CRPS) is a painful disorder of the extremities, characterised by sensory, autonomic, vasomotor, motor and trophic disturbances (see Figures 1 and 2). CRPS mostly occurs after a trauma, such as a fracture or an operation, but can also develop without a preceding event.1,2 In the Netherlands, approximately 4,300 patients develop CRPS each year, whereby females are affected three times more than males and the highest incidence is found between the age of 61 and 70 years.3
Diagnosing Complex Regional Pain Syndrome
Complex regional pain syndrome (CRPS) is a painful disorder of the extremities, characterised by sensory, autonomic, vasomotor, motor and trophic disturbances (see Figures 1 and 2). CRPS mostly occurs after a trauma, such as a fracture or an operation, but can also develop without a preceding event.1,2 In the Netherlands, approximately 4,300 patients develop CRPS each year, whereby females are affected three times more than males and the highest incidence is found between the age of 61 and 70 years.3
Diagnosing Complex Regional Pain Syndrome
The diagnosis of CRPS is based on clinically observed signs and symptoms reported by the patient. Additional laboratory or radiological assessments provide insufficient basis for diagnosing CRPS, but should be used to exclude other pathologies (such as an unresolved fracture or active infection).4 Several sets of diagnostic criteria have been proposed over the past decades, some of which are still being used concurrently. The criteria of Veldman et al.5 are based on the identification of a limited amount of signs and symptoms, which are present predominantly in the acute phase of CRPS. The International Association for the Study of Pain (IASP)-Orlando criteria6 allow for the diagnosis to be made almost exclusively based on anamnestic information and appear to be more sensitive than the Veldman et al. criteria.7 More specific criteria have been developed by Bruehl and Harden8 requiring both anamnestic and observed information regarding sensory, vasomotor, motor, sudomotor and motortrophic disturbances. An adapted version of the latter criteria set has been validated internationally, resulting in a diagnostic tool that combines good specificity with excellent sensitivity for diagnosing CRPS: the Budapest criteria (see Table 1).9 These criteria have recently been adopted by the IASP as the international standard for diagnosing CRPS.
To maximise the comparability of studies of CRPS and ensure agreement between clinicians involved in diagnosing and treating CRPS, a uniform and internationally accepted criteria set such as the Budapest criteria is necessary. Uniform diagnosis and assessment of CRPS could be further improved by identification of disease markers of CRPS Type 1 (CRPS-1) and development of objective assessment tools.
Pathophysiological Mechanisms of Complex Regional Pain Syndrome
Neurogenic and immune-mediated inflammation, disproportional oxidative stress, autonomic dysfunction, vasomotor dysfunction, increased neuronal excitation, central sensitisation, cortical reorganisation and psychological predisposition have been proposed as possible disease mechanisms for CRPS. This variety in pathophysiological perspectives combined with possible simultaneous occurrence of different mechanisms in a single patient, might provide an explanation for the heterogeneity of phenotypes described in CRPS literature in recent years.
Neurogenic Inflammation
A trauma can easily result in micro-injury of small nerve fibres, which in turn triggers the release of neuropeptides, such as substance P (SP) and calcitonin gene-related peptide (CGRP) in the periphery.10,11 This excessive release of neuropeptides, called neurogenic inflammation, induces vasodilatation and increases vascular permeability, leading to plasma extravasation and attraction of immune mediators to the site of injury, resulting in an inflammatory response. Neurogenic as well as immune-mediated inflammation contribute to the generation of pain, whereby features of neuropathic sensitisation, such as allodynia or hyperesthesia, are observed. In patients with CRPS, elevated levels of CGRP have been found, suggesting a contribution of neurogenic inflammation to the development and maintenance of in this condition.12
Immune-mediated Inflammation
Especially during the early stages of the disease course, CRPS signs and symptoms resemble the classic clinical presentation of inflammation: rubor, calor, dolor and functio laesa. Increased levels of inflammatory markers and markers of mast cell activity have been found in blister fluid (e.g. interleukin [IL]-6, tumour necrosis factor [TNF]-α and tryptase) and serum (IL-8, soluble TNF receptors and SP) obtained from the affected extremity of patients with CRPS, compared with both the unaffected extremity and healthy controls.10,13,14 Indications for central inflammatory activity can be found in increased pro-inflammatory cytokines (IL-6) and decreased anti-inflammatory cytokines (IL-4 and IL-10) in cerebrospinal fluid.15 Furthermore, markers of increased glial cell activation (glial fibrillary acidic protein [GFAP] and monocyte chemoattractant protein 1 [MCP1]) found in CRPS, might provide support for neuronal-driven immune activation.16
Oxidative Stress
Excessive, non-self-limiting release of free oxygen radicals leading to oxidative stress has been proposed as a pathophysiological mechanism underlying CRPS. Studies based on animal models have shown that infusion with free radical-inducing agents results in features that resemble clinical signs of CRPS, such as swelling, impaired function, increased temperature, redness and increased pain sensitivity.17 A role for increased oxidative stress is also supported by the observation of elevated levels of oxidative markers in the serum and saliva of patients with CRPS-1.18 Furthermore, leukocyte accumulation in the affected extremity has been observed in patients with CRPS, probably resulting from increased vascular permeability owing to oxidative stress.19 Tissue hypoxia, as shown by the poor oxygenation of skin in patients with CRPS,20 lends further support to this observation. The efficacy of free radical scavengers, such as dimethylsulfoxide and N-Acetylcysteine,21,22 in the treatment of CRPS-1 and the preventive effect of vitamin C after fractures23,24 in the development of this disease provide indirect evidence for the oxidative stress hypothesis.
Autonomic Disturbances
Hyperactivity of the sympathetic nervous system has long been thought to be the primary pathophysiological mechanism of CRPS-1, resulting in increased vasoconstriction, increased sweating and trophic disturbances. However, studies have shown lower levels of sympathetic neurotransmitters in the affected limb25–27 compared with the unaffected limb, indicating decreased sympathetic activity. Presumably, increased sensitivity of α-adrenergic receptors, probably resulting from reduction of sympathetic neural traffic, would explain this phenomenon.28 The extent in which autonomic disturbances are observed can differ depending on the stage of the disease course.29
Vascular Dysfunction
An alternative hypothesis for the vasomotor instability observed in patients with CRPS is based on endothelial dysfunction resulting in hypoxia, a decrease in nitric oxide (NO) synthase and an increase in endothelin-1.30 This dysfunction leads to clinical features, such as a cold affected extremity and discolouration (pale, blue skin), and other features of CRPS associated with oxygen deprivation.
Neuronal Excitation and Central Sensitisation
Clinical features displayed in CRPS, such as allodynia, hyperalgesia and wind-up, have been related to the process of central sensitisation.31 This process is triggered by the release of SP, CGRP and glutamate after tissue damage, which in turn activate the normally dormant N-methyl-D-aspartic acid (NMDA) receptor.32 Elevated levels of glutamate found in the serum and cerebrospinal fluid of patients with CRPS are suggestive of the involvement of NMDA receptor responses.33 Central sensitisation might also influence the spinal motor circuitry, resulting in movement disorders associated with CRPS, such as dystonia, tremor or myoclonia.34
Cortical Reorganisation
Pain and sensory disturbances in CRPS often spread from the location of the initial trauma to a larger area, sometimes even to another extremity, which might indicate plastic changes of the central nervous system owing to neurogenic inflammation.35 In patients with CRPS, reorganisation of the primary somatosensory cortex (S1) has been observed, which correlated with the amount of pain and hyperalgesia experienced by the patients.36,37 Cerebral representation and motor processing in the brain are also reported to be disturbed, possibly leading to movement disorders in CRPS-1 and distorted visualised representation of the affected limb.38–41
Psychological Factors
Psychological disturbances have often been proposed to be involved in complex conditions, such as chronic pain and CRPS. However, little evidence has emerged to support this hypothesis. No relation has been found between psychological dysfunction, disease-related fear or personality and the development of CRPS.42–44 One study reports that stressful life events are more common in patients with CRPS than in controls,45 but other studies could not confirm this finding.42,43,46,47 Once patients have developed CRPS, pain-related fear and fear of re-injury are proposed to be risk factors for a poor prognosis relating to pain reduction and functional improvement.48,49
Treatment Options
Treatment of CRPS-1 is challenging, because of the variety of symptoms and the variable disease course exhibited by patients. A multimodal approach consisting of pharmacological treatment and physiotherapy, sometimes in combination with invasive therapy or psychological support, is required. An overview of systematic reviews and guidelines providing an evidence-based approach to treatment of CRPS is presented in Tables 2 and 3.50–60
Pharmacological Treatment
Analgesics
Pain medication according to the WHO analgesic ladder has been suggested in therapeutic guidelines, although evidence supporting the efficacy of paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDS) is limited. Tramadol has been shown to be effective in neuropathic pain disorders, therefore it can be considered for severe pain accompanying CRPS, although evidence for its effects in CRPS is lacking.4,50,51,55,61–63
Treating CRPS with strong opioids should only be considered as crisis management for a limited period of time.51,55 Furthermore, gabapentin has proven efficacy in CRPS-1;50,51,55,62,64 however, amitryptiline and carbamazepine or newer tricyclic antidepressants (TCAs), such as duloxetine or venlafaxine, can also be considered, because of their shown effectiveness in other neuropathic pain disorders.50,55,62
Intravenous administration of the NMDA receptor antagonist ketamine can be considered; however the full scope of its therapeutical potential (including a risk–benefit assessment) has not yet been established.50,60,65,66 Lidocaine patches have been proposed for treatment of localised sensory deficits, such as allodynia in CRPS.67
Anti-inflammatory Therapy
The free radical scavenger dimethyl sulfoxide (DMSO) has been shown to be effective in patients who have had CRPS-1 for less than a year.58 N-Acetylcysteine (NAC) shows comparable efficacy to DMSO, but proved superior to DMSO for primary cold CRPS in subgroup analyses.68 Vitamin C has established efficacy in the prevention of CRPS after wrist fractures.23,24 Furthermore, corticosteroids can provide significant pain reduction in CRPS; however, there is no consensus on the dosage or duration of treatment.50,58,62,63 Both types of intervention have so far only been evaluated in early-stage CRPS.51,55
Calcium-regulating Drugs
The use of bisphosphonates has been proposed as a treatment option for CRPS. Although limited support for their ability to reduce pain (associated with bone loss) has been reported, additional research with regard to their dosage, frequency and duration of treatment is required.21,50,53,55,56,62,69,70
Vasodilatory Medication
For treatment of patients with CRPS and vasomotor disturbances, α-1 adrenergic blockers, phenoxybenzamine and terazosin, or calcium influx blockers, such as nifedipine, can be considered.4,55,71 No reduction in temperature asymmetry was found for NO-regulating medication (e.g. tadalafil or isosorbide dinitrate) in primary cold CRPS, although tadalafil is superior to placebo in reducing pain for this subgroup.72,73 Likewise, intravenous administration of ketanserine is reported to reduce pain in CRPS.50,74
Spasmolytics
Movement disorders in CRPS-1, such as dystonia, myoclonia and tremor, might benefit from treatment with baclofen or benzodiazepines.4,50,63 Treatment with anti-cholinergics has not shown to be beneficial for CRPS-related movement disorders over a longer period of time.34
Physical Therapy
An important modality for treatment of CRPS is physical therapy directed at increasing control over pain and improving skills.57,75 Motor imagery and mirror therapy have been proven effective, both of which are applied to counter disturbed cortical motor processing, resulting in improvement of pain and function.41,76,77 Transcutaneous electrical nerve stimulation (TENS) might be beneficial for treatment of pain in a subgroup of patients and might therefore be a suitable adjunctive non-invasive therapy. However, evidence for the latter is limited.4
Invasive Treatment
Spinal cord stimulation with an implantable generator can be considered for patients with chronic CRPS; however, the high incidence and severity of complications following spinal cord stimulation warrant careful patient selection.78 Spinal cord stimulation should not be considered in early stages of CRPS.79 Studies of intrathecal administration of baclofen show that patients with CRPS dystonia can experience marked improvement in pain and disability levels, paralleled by improvement in quality of life.80,81 However, as with other intrathecal approaches, complications can be severe and, therefore, should be limited to patients who are refractory to conventional therapy and be conducted by physicians with ample experience with intrathecal devices.81
Psychological Treatment
Although studies with regard to psychological interventions for CRPS are limited, treatment by a psychologist can be considered in cases where disease burden is high or there is a discrepancy between noted pain behaviour and observed signs and symptoms of CRPS.63 Graded exposure is a promising therapy to reduce fear of pain and to regain functionality of the affected extremity.49
Future Perspectives
In recent decades, much research effort has been directed to unravelling the underlying mechanisms of CRPS, and improving strategies for its prevention and treatment, alongside the unification of diagnostic procedures. An important issue to be addressed is the identification of prognostic factors for disease development, which could lead to a more targeted approach and therewith improve the prognosis of patients with CRPS. Prospective cohort studies on the development of CRPS are necessary to gain a better understanding of prognostic factors related to disease onset and disease course.82 The recently developed CRPS severity score (CSS) as a derivation of the Budapest diagnostic criteria83 might be helpful in improving the systematic follow up of patients; however, validation of this tool is ongoing. CRPS remains a clinical diagnosis, and the patient population is heterogeneous. Although it has been proposed that CRPS comprises different disease subtypes or stages of the disease, this has not led to further subcategorisation of the disease.8 A targeted approach based on the identification of CRPS subtypes and specific mechanisms prevailing in an individual patient is therefore still warranted.
The many available treatment options suggest that the optimal therapy for CRPS has not yet been identified. Given the heterogeneous nature of CRPS, an optimal therapy seems unlikely; therefore, a mechanism-directed approach to treatment of CRPS appears preferable. With regard to interventions targeting inflammation, comparative studies of established interventions (e.g. prednisolone and DMSO) and novel anti-inflammatory agents, such as intravenous administration of immunoglobulin,84 should be performed. Furthermore, alternative approaches, such as targeting the cholinergic anti-inflammatory pathway,85 whereby activating the parasympathetic nervous system to inhibit inflammatory activity and autonomic dysregulation in CRPS, could be pursued. Promising interventions addressing sensory disturbances related to peripheral and central sensitisation, such as NMDA receptor antagonists86 and the N-type calcium channel blocker ziconotide,87 are worthwhile targets for further research.
Therapy directed at the stimulation of adaptive cortical reorganisation involving brain-training programmes, such as mirror therapy76 and motor imagery,77 merit implementation in daily practice. Continuing this line of thought, a strong point can be made for increasing patients’ awareness and knowledge regarding mechanisms underlying the development of chronic pain and CRPS.88 Further research within the field of exercise and occupational therapy should be focused on the distinction between pain and time-contingent approaches. Positive initial results have been found for pain exposure physical therapy (PEPT), which is based on progressive-loading exercises and desensitisation beyond the patients’ pain limits.89 Cognitive behavioural aspects are taken into account, with the goal to motivate patients to use the affected limb in daily activity despite experiencing pain. Likewise, for patients with CRPS with pain-related fear, research is ongoing into the effects of graded exposure therapy (GEXP), comprising provision of information about CRPS, observational learning and ‘flooding’ of feared movements and activities.
Repetitive transcranial magnetic stimulation (rTMS), whereby the motor cortex is stimulated to treat neuropathic pain, has been shown to provide short-term pain relief in patients with CRPS-1.90 However, issues related to placebo response and ways to prolong its efficacy need to be addressed.
For each of the interventions discussed in this article, long-term studies of sufficient sample size, with specific attention to patient selection, timing and dosage of therapies, are required to establish risks and benefits of each intervention for patients with CRPS. ■