Peripheral nerve stimulation (PNS) is a unique neuromodulation modality that is rapidly gaining popularity for a variety of clinical conditions. Despite its straightforward nature, the modality has been for a long time treated as a ‘stepchild’ of the neuromodulation field, yielding the spotlight to the more ubiquitous spinal cord stimulation (SCS) and the more elegant deep brain stimulation (DBS) approaches. Interestingly enough, the main theoretical explanation of the neuromodulatory treatment of pain, the ‘gate control theory’ of Melzack and Wall1 was first illustrated by an example of PNS when courageous investigators were able to suppress pain perception with electrical stimulation of their own infraorbital nerves using percutaneously inserted electrodes.2
Since its introduction in the late 1960s, PNS went through several stages of development.3 Although there were many enthusiastic centers and clinical series describing the use of PNS in a variety of neuropathic pain conditions, the modality did not become popular for more than 30 years. The complexity of PNS procedures, with the need to expose the targeted nerve and secure the stimulating electrode, and the unpredictability of PNS outcomes, along with the lack of dedicated and approved PNS equipment, resulted in its lack of widespread acceptance. However, even in the 1970s and 1980s, several large series showed the usefulness of PNS in the treatment of neuropathic pain syndromes, including chronic pain associated with peripheral nerve injury and complex regional pain syndromes.4–9
The situation changed in the late 1990s when a percutaneous PNS approach was described by Weiner and Reed.10 The simplicity and elegance of a surgical procedure that did not require major exposure to achieve direct contact between the nerve and electrode lead, but instead allowed one to put the electrode in the vicinity of the nerve to be stimulated, revolutionized the field. Among other implications, the introduction of a percutaneous approach gave an opportunity to use PNS to pain specialists with a background in anesthesia or physiatry, whereas in the past this modality was only available to neurosurgeons and orthopedic and plastic surgeons who felt comfortable exposing and dissecting peripheral nerves.
Definition of Peripheral Nerve Stimulation
PNS in its pure sense has traditionally referred to a modality where electrical impulses are delivered directly to the peripheral nerve. In the past, this involved surgical exploration of the nerve and placing an electrode array directly on it—using either a flat paddle that touched the nerve or was separated from it by a thin fascial layer, or a spiral electrode that could be wrapped around the nerve trunk. Independently of the electrode/nerve interface, the principle of PNS remains the same, and when stimulation is applied, the paresthesias (pleasant sensation described as tingling or warmth) are felt in the distribution of sensory representation of the stimulated nerve.
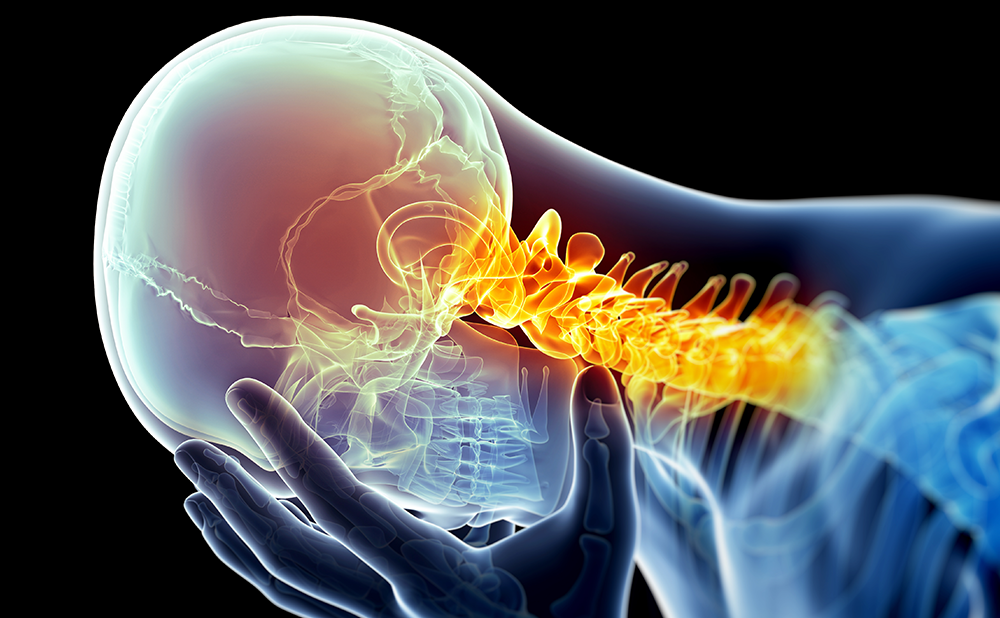
In a similar fashion, modern applications of PNS in the treatment of pain produce paresthesias in the distal body regions that represent the sensory coverage of the nerve. The best examples of such a principle would be stimulation of the occipital nerves, when paresthesias are perceived all the way to the vertex of the skull or the frontal hairline while the electrode is located over the craniocervical junction, or infraorbital nerve stimulation, which produces paresthesias in the upper teeth and upper lip while the electrode is traveling next to the infraorbital foramen. In contrast to true PNS, the more recently introduced concepts of subcutaneous neuromodulation,11 subcutaneous peripheral neurostimulation (SPNS),12 peripheral nerve field stimulation (PNFS),13 peripheral subcutaneous neurostimulation (PSNS),14 and peripheral subcutaneous field stimulation (PSFS)15 refer to stimulation of smaller nerves or even nerve endings and the paresthesias are felt in the vicinity of the electrode itself. Since these concepts (PNS and PNFS/SPNS/PSNS/PSFS) may overlap to some extent, and since none of them currently has regulatory approval in the US (European approval was obtained in 2010 by two different companies), there is a certain element of confusion in the literature regarding terminology. This issue has already been addressed in multiple publications16–18 but continues to be a point of contention at professional conferences, as there is no universal agreement on where to draw the line (and whether the line has to be drawn) between these two approaches.
Mechanism of Action
Interestingly enough, despite a long history of clinical application of PNS, its mechanism of action remains rather unclear.19 An original explanation, the cornerstone of the gate control theory of pain, postulated that antegrade (orthodromic) stimulation of non-nociceptive Aβ nerve fibers results in activation of the same interneurons in the superficial layers (Rexed laminae 2 and 3) of the dorsal horn of the spinal cord that are involved in processing and transmission of nociceptive information delivered by peripheral Aβ and C nerve fibers. Such non-painful stimulation provided by PNS inhibits the interneurons and interrupts or decreases transmission of pain signals.1 Additional modifications of the half-century-old theory enhanced it by more complex excitatory and inhibitory interactions but the general principle remains the same.20 Another possible explanation combines electrical and neurohumoral effects of stimulation, as peripheral stimulation may be changing local concentrations of important chemicals, such as neurotransmitters and endorphins, and augments local blood flow that may be contributing to production of chronic pain.5,19
In addition to this, PNS may directly change the excitability of peripheral nerve fibers.21 A recent experimental study on human volunteers showed that direct stimulation of a peripheral nerve inhibits neurotransmission, as documented by elevated thresholds for nociceptive stimulation.22 The mechanism of pain suppression, however, is likely to be more complex than simple peripheral and spinal inhibition. Multiple neuroimaging studies convincingly indicate the presence of central mechanisms of PNS action. These include both suppression of activity in pain-processing cerebral circuits and activation of those areas that are involved in the descending system of pain control and modulation.23
Common Indications
The simple fact that the peripheral nervous system supplies the entire human body translates into the applicability of the PNS approach to essentially every localized pain syndrome. Over the years, PNS has been successfully used in treatment of pain in both upper and lower extremities,24 in the truncal area, including the lower back, abdomen, and inguinal region, in the chest wall anteriorly and posteriorly (post-sternotomy pain and intercostal neuralgias),25,26 and in the craniofacial region.10,27–29
The original PNS use was aimed at patients with painful peripheral neuropathies in the extremities, mainly due to traumatic injury of the nerve that was subsequently chosen as a target for stimulation. This evolved to acceptance of PNS in the treatment of chronic pain due to post-surgical or entrapment neuropathy, as well as complex regional pain syndromes, both type 1 (formerly known as reflex sympathetic dystrophy) and 2 (causalgia).30–36 Chronicity and severity of pain, as well as failure of less invasive approaches, have been established as necessary criteria in patient selection. It became clear early on that the best responders to PNS are those patients whose pain is mediated by primarily sensory nerves, since mixed and predominantly motor nerves do not tolerate PNS well, as motor phenomena due to stimulation prevent the increase in amplitude required for pain suppression.8
These major peripheral nerves traditionally had to be exposed surgically, not only because of their deep course within soft tissues, but also because of the frequent proximity of vascular structures. The issue of localization of the nerve trunks and delineation of adjacent vascular structures was resolved with the introduction of ultrasound guidance during percutaneous PNS electrode insertion.37,38 The rebirth of open surgery for very specific cases of pain due to peripheral nerve injury—those caused by the presence of post-amputation neuromas—is expected with the development of a new dedicated PNS system with special cuff-like electrodes that is now undergoing clinical testing (unpublished data).
In treatment of chronic pain in the extremities, the open surgical approach remains the ‘gold standard’ in reaching the peripheral nerves, as evidenced by recently published large clinical series from Israel and Australia35,36 and a uniquely long follow-up in a cohort of patients implanted in the 1980s in Belgium.39 For this application, a percutaneous approach is only starting to gain acceptance as evidenced by anecdotal reports using PNS in both upper40,41 and lower37,38,42 extremities. Chronic neuropathic pain in the neck, chest, abdomen, lower back, and pelvis has been successfully treated with PNS and PNFS applications. There are published reports of PNS/PNFS use in localized neck pain;14,15 chest wall pain due to intercostal neuralgia43 and after sternotomy,44 thoracotomy,45 scapular fracture,46 and thoracic myelopathy;47 abdominal13,48 and inguinal49 pain; lower back pain—with the use of percutaneous electrodes implanted close to the area of pain,12,50 percutaneous electrodes implanted far from each other (the so-called ‘cross-talk’ concept),51 a combination of epidural and peripheral electrodes,25,52 and paddle electrodes;53 and pelvic pain and coccygodynia.54
Lower back pain, being the most prevalent of the truncal pain syndromes, was a subject of a recent multicenter outcome study performed on a nationwide scale in Austria.55 In this study, the clinical outcomes of 111 patients with focal, non-cancer pain were retrospectively analyzed for at least three months after permanent implantation. Sixty-six of these 111 patients had either lower back pain or back pain with leg pain due to failed back surgery syndrome; the rest suffered from neck pain, post-herpetic neuralgia, thoracic back pain, tension headache, and facial pain. The overall improvement of pain was more than 50 % (reduction from 8.2 to 4.0 on a numerical rating scale of 0 to 10) for the entire group. In addition to this, the group of stimulated patients experienced sustained reduction in demand for analgesics.55 Despite the retrospective nature of this study and a 24 % observed complication rate,55 its results, along with another study that investigated the efficacy of combined SCS and PNFS in the treatment of lower back and leg pain in a series of 20 consecutive patients,52 allowed Medtronic to receive the Conformité Européenne (CE) mark for its 16-electrode, fully implantable system for the percutaneous delivery of PNS for the management of chronic back pain in May 2011.
Not surprisingly, a major shift in indications for PNS coincided with the introduction of percutaneous insertion techniques, when treatment of craniofacial pain syndromes became amenable to PNS interventions. Prior to that, exposure of trigeminal branches56 and occipital nerves7,9,27 that provide sensory supply to the head and face region was technically challenging. But when placing an electrode became as simple as insertion of a guiding needle into the epifascial plane,10 many centers started using percutaneous PNS for treatment of all kinds of neuropathic pain in the craniofacial region.57–61 With mostly anecdotal reports, various indications, such as supraorbital neuralgia, infraorbital neuropathic pain, post-herpetic neuralgia in the ophthalmic nerve distribution, and occipital pain due to post-surgical neuroma, were explored. Based on this experience, larger series of patients were collected and presented. These included trigeminal neuropathic pain,62–64 occipital neuralgia treated with percutaneous65 and paddle-type electrodes,66–68 and, finally, chronic cervicogenic headaches and migraines.29,66,69–72
The use of PNS for migraines that started with the publication of Popeney and Aló69 immediately attracted the attention of both the implanting community and the device manufacturers, as the prevalence of migraines and percentage of medically intractable cases make this indication potentially larger than all other current indications together (with the exception of another potential PNS indication—lower back pain). Subsequently, all three large neuromodulation companies (Medtronic Neuromodulation, Minneapolis, MN; St Jude Neuromodulation, Plano, TX; and Boston Scientific Neuromodulation, Valencia, CA) started prospective controlled studies investigating the feasibility and efficacy of occipital nerve stimulation in the treatment of intractable migraines.73–75 The results of all three studies were positive overall and, in the end, PNS became approved for treatment of intractable migraines in Europe (at the time of writing, European CE Mark approval was received by St Jude Medical for its GenesisTM neurostimulation system for PNS of the occipital nerves for the management of the pain and disability associated with intractable chronic migraine).
In addition to occipital PNS, migraines have been successfully treated with a combination of supraorbital and occipital electrodes76,77 and, more recently, with bilateral auriculotemporal nerve stimulation.78 The rationale here is that stimulation appears to be most effective when applied to the area of maximal pain, and therefore some patients would benefit more from an individualized approach to PNS electrode insertion as the headache location in migraine sufferers tends to involve different parts of the head.
Less common, but perhaps even more resistant to treatment, and definitely more disabling overall, conditions treated with PNS are cluster headaches and hemicrania continua. Both of these pain categories were successfully treated with occipital nerve stimulation,79–84 supraorbital PNS,85, vagus nerve stimulation,86 and, more recently, with sphenopalatine ganglion stimulation.87 Here PNS may be a less invasive alternative to the earlier-introduced hypothalamic DBS and perhaps serve as a first step in neuromodulatory treatment, leaving DBS as an option for PNS failures.
Finally, the latest, and perhaps most unexpected, PNS indication in the treatment of pain became diffuse pain of fibromyalgia.88,89 Here, in a first group of 12 patients satisfying the criteria for fibromyalgia, occipital PNS was implanted for control of occipital headache—and, in addition to improvement in headache severity, patients were found to have significant improvement in scores for bodily pain, depression, fatigue, and quality of life.88 Following this success in a prospective uncontrolled cohort, the same group of investigators implanted another 11 fibromyalgia patients and then stimulated them in a placebo-controlled cross-over fashion.90 The results of this study revealed that nine of the 11 patients proceeded with permanent implantation and that at six-month follow-up there was an overall 45 % decrease in pain and a significant decrease in the amount of positive trigger points and the overall score on the fibromyalgia impact questionnaire, making the authors conclude that the results of the original prospective study had been confirmed in a placebo-controlled manner.90
Technical Details and Complications
The most attractive part of the PNS approach is its low invasiveness. Instead of putting electrodes into or over the patient’s brain, as in DBS or motor cortex stimulation, or into the spinal epidural space, as in SCS or nerve root stimulation, the entire electrode array is positioned in the vicinity of the stimulated peripheral nerve. In the case of PNFS, the electrode is placed subcutaneously in the region of maximal pain. Such an approach inevitably translates into increased safety—and, although there are many reports describing various complications that arise from the use of PNS and PNFS procedures,91–95 most of these complications are minor and do not represent any threat to the life or neurological function of the patient operated on.
As a matter of fact, the most likely reason for the higher PNS complication rate is the lack of dedicated hardware, as PNS for treatment of chronic pain is mainly performed with devices developed and marketed for SCS applications.95 Nevertheless, it is worth mentioning that patients should be routinely informed that their PNS systems may malfunction, the hardware components may become broken or disconnected, they may erode through the skin and/or become infected, and electrodes may provoke muscle spasms or migrate from their optimal location necessitating reprogramming or repositioning. It appears that some of these complications may be resolved without additional surgery, but even in those cases where surgery is needed to revise, replace, or remove an affected device, it can be safely done on an outpatient basis with minimal, if any, morbidity.
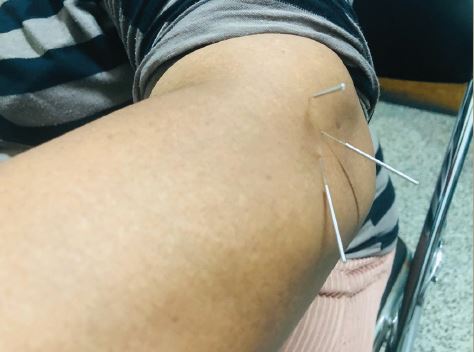
Most percutaneous electrodes used today have either four or eight cylindrical contacts placed along a single wire-like lead. In the future the number of contacts is expected to grow to 12 or even 16, thereby increasing the length of potential stimulation targets. These percutaneous electrodes may be implanted through a needle that is inserted under the skin in an epifascial plane. Paddle electrodes are larger and generally more stable; here, the flat metal contacts (four, eight or 16 per paddle) are positioned on a hard plastic backing that insulates the surrounding tissues. Among their benefits are a lower incidence of migration and the unidirectional nature of stimulation that aims electrical signals toward the stimulated nerve and away from adjacent sensitive tissues. These electrodes, however, usually require open surgical exposure of the nerve or stimulated area; they are also harder to remove if such removal is required in the future.
Similarly to the SCS procedure, PNS and PNFS start from careful patient selection and mandatory (in most practices) psychological evaluation. The final step before permanent device implantation is the stimulation trial when temporary electrodes are inserted and tested for efficacy and side effects. Such a trial usually lasts between three and seven days, although in some centers, particularly in European countries, it may last up to two months. During the trial period, patients determine the benefits of stimulation, helping them gauge expectations and determine whether permanent implantation is warranted. For the entire duration of the trial the patients remain connected to an external screening device that has most features of the stimulation that will be delivered by the implanted generator if the trial succeeds. At the end of this trial, temporary electrodes are removed (if the trial fails) or get replaced with permanent ones (if the trial was successful). Permanent PNS/PNFS electrodes are anchored in place and then tunneled toward an implantable pulse generator (IPG) that is usually placed at some distance away from the stimulated region. The IPG device may have a primary cell or be rechargeable—this rechargeability allows a reduction in device volume and an extension of its longevity, but usually comes at a slightly higher price and requires active participation/maintenance from the patient, who has to recharge the IPG on a regular basis. There are many devices and hardware combinations on the market today allowing implanters to come up with individualized sets of electrode leads and generators depending on pain location, stimulation requirements, patient characteristics, etc.95
Future Directions
It appears that PNS is today the most rapidly growing field of neuromodulation.96 With the recent regulatory approval of PNS in Europe for the treatment of chronic lower back pain and intractable migraines, it is expected that clinical interest in this modality will continue to rise. Among other things, this carries a hope that there will now be some much-needed objective evidence that determines the true efficacy of PNS and the best indications and most appropriate parameters of stimulation. Associated research activity may also shed some light on the PNS mechanism of action which will then translate into further individualization of treatment and through it into optimization of immediate and long-term outcomes. Moreover, among important questions that remain open is the issue of the cost-effectiveness of PNS, as the high cost of implanted devices has to be justified in the view of ongoing efforts to contain healthcare costs.
As to progress in the PNS field itself, it appears that it will continue in all three directions—new indications, new targets and new devices—each of which deserves a separate article due to the unlimited opportunities that these directions represent. One of the main turning points in this process will be official endorsement of the entire approach through its regulatory approval, not only in Europe but worldwide, particularly in the US, which represents the biggest neuromodulation market today. Such endorsement will allow implanters to use approved devices for approved indications—instead of doing it on an ‘off-label’ basis—and at the same time will give device manufacturers a chance to market these devices and support education on their rational use.