Ischaemic stroke may occur due to several potential mechanisms. Mechanisms are classically described as large artery atherosclerosis, small-vessel occlusion, cardioembolism, stroke of other determined aetiology (i.e. vasculitis, genetic disorder, etc.) and stroke of undetermined aetiology. Historically, the definition of stroke of undetermined aetiology has included strokes with multiple potential mechanisms, as well as those with negative or incomplete evaluations.1 Over time, as the approach to stroke evaluation has evolved, the term cryptogenic stroke has largely been reserved for strokes with no identified cause, despite adequate standard evaluation.1–3 Since most cryptogenic strokes are thought to be embolic, the term embolic stroke of undetermined source (ESUS) has also become popular, and is often used synonymously with cryptogenic stroke. ESUS, specifically, is defined as a stroke not attributed to a lacunar mechanism (i.e. >2.0 cm), not associated with ≥50% intracranial or extracranial arterial stenosis, and not due to a major source of cardioembolism, such as atrial fibrillation.1–4
An estimated 20–30% of all strokes will have no probable cause despite appropriate initial testing, which equates to 150,000–240,000 cryptogenic strokes per year in the United States.2,3 Typically, patients with cryptogenic stroke are younger and have fewer traditional vascular risk factors.4,5 Numerous mechanisms have been proposed for cryptogenic stroke, including cardioembolism from occult atrial fibrillation, cardiac structural abnormalities, paroxysmal embolism, hypercoagulable or prothrombotic states, sub-stenotic large-vessel disease, aortic atherosclerotic disease and other non-atherosclerotic vasculopathies.6 Determining the underlying mechanism is important, since cryptogenic strokes are associated with increased risk of recurrent stroke compared with strokes due to a defined mechanism. Knowing the source of stroke allows for the best treatment to prevent secondary strokes.5,7 Herein, we discuss the standard evaluation of ischaemic stroke, potential causes of cryptogenic stroke and recommended additional evaluations to better identify stroke mechanism.
Initial evaluation of ischaemic stroke
Though the approach to stroke workup should be individualized and adjusted to the age, gender and specific risk factors of each patient, certain studies should almost always be performed. In general, the initial goal of testing is to assess for one of the three common mechanisms of stroke: large-vessel arteriosclerotic disease, small-vessel disease and cardioembolism.1 Initial evaluations should include brain and vessel imaging, echocardiogram, cardiac monitoring and basic blood tests.6 Though computed tomography (CT) of the head is an option and often the first study performed during assessment in the acute setting of suspected stroke, magnetic resonance imaging (MRI) of the brain is often far superior in detecting abnormalities and is often warranted. An MRI will better evaluate the stroke topography, which often can provide clues into potential mechanism. For example, multiple acute strokes within different vascular territories are worrisome for proximal or cardioembolic source, whereas a small stroke within the deep structures of the brain, which is also associated with a significant burden of white matter disease, is more consistent with a lacunar infarct due to small-vessel pathology. Carotid ultrasound (US), CT angiography (CTA) or MR angiography (MRA) of the neck are helpful in evaluating for large-vessel atherosclerotic disease within the neck. CTA or MRA of the head is also highly recommended given the potential for intracranial disease as a mechanism of stroke that would not be readily appreciated on neck vessel imaging.1–7
At a minimum, an electrocardiogram (ECG) and inpatient cardiac monitoring should be performed on all patients admitted with stroke/transient ischaemic attack (TIA). In those without clear small-vessel mechanism, an additional 24 hours of cardiac monitoring is recommended to evaluate for atrial fibrillation. Typically, a transthoracic echocardiogram (TTE) or transoesophageal echocardiogram (TOE) is recommended to identify potential structural cardiac sources of stroke, such as intracardiac thrombus or valvular disease. Basic laboratory tests should be performed to identify underlying coagulopathy and to assess for common modifiable risk factors of stroke (i.e. diabetes, hyperlipidaemia and thyroid disease). If these initial studies do not reveal stroke mechanism, the stroke is assumed cryptogenic and further evaluations are warranted (Figure 1).
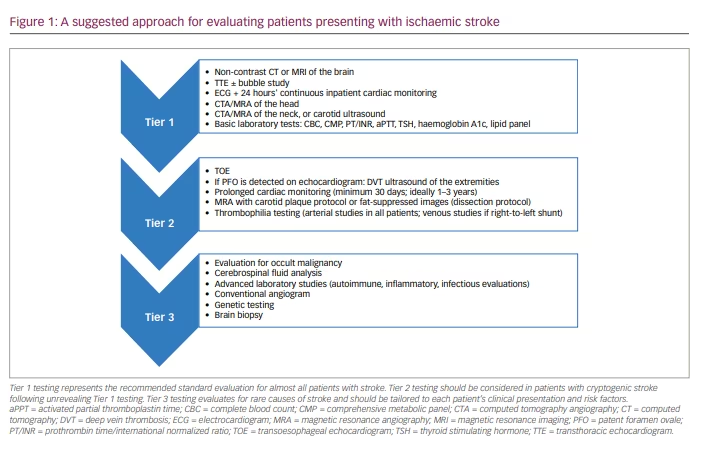
Potential causes of cryptogenic stroke
Occult atrial fibrillation
Occult atrial fibrillation is the likely cause of up to 30% of cryptogenic strokes. It is typically asymptomatic and paroxysmal, making detection difficult. However, detecting the arrythmia is important as its treatment, anticoagulation, ultimately improves secondary stroke prevention.8,9
The standard stroke evaluation typically includes 24-hour cardiac monitoring, but the sensitivity of capturing atrial fibrillation during this period is low.10 Thus, patients with cryptogenic stroke warrant more prolonged monitoring. Multiple devices are available with variable durations of cardiac monitoring as options. Not surprisingly, longer periods of continuous monitoring are consistently associated with higher rates of detection of atrial fibrillation, and implantable cardiac monitors have the highest rate of detection of all devices.8,11–13 In fact, 40% of patients with cryptogenic stroke with implantable cardiac monitors were found to have atrial fibrillation, with a mean time to detection of 8 months.14 Older patients (>75 years old), patients with ejection fraction (EF) <40%, and those with prolonged PR interval on initial ECG have higher rates of detection.9,10
TTEs and, especially, TOEs can also provide clues of the risk of underlying atrial fibrillation. Enlargement of the left atrium and reduced left atrial emptying velocities are findings of particular concern. Even more concerning are findings of thrombus, ‘smoke’ and ‘spontaneous echo contrast’ within the left atrium and/or left atrial appendage. Patients with such findings are at particularly high risk of stroke due to atrial fibrillation, and anticoagulation should be seriously considered for secondary stroke prevention.10–12
Patent foramen ovale
Patent foramen ovale (PFO) is a relatively frequent inter-atrial communication found in approximately 25–30% of the general population, and is often clinically insignificant.13–15 However, a higher frequency of PFO is found in patients with cryptogenic stroke, raising the question of its potential role in stroke pathogenesis.13 Potential mechanisms of stroke in patients with a PFO include paroxysmal embolism from a venous source or embolization of thrombotic material formed on the PFO, or associated atrial septal aneurysm (redundant and mobile inter-atrial septal tissue).14
TTE and TOE with saline contrast injection are often used to detect PFOs. TOE is considered the gold standard for detection with sensitivity of approximately 90% and specificity of 95%.6,15,16 However, older patients or patients with severe dysphagia may be less able to tolerate the procedure. Further, sedation used during the procedure may limit the effectiveness of Valsalva manoeuvres, which aid in diagnosis. A TTE with bubble study is an alternative, less invasive means of detecting PFOs, though the sensitivity and specificity are lower than TOE.13 Transcranial Doppler ultrasonography with bubble contrast material can also be used to improve detection of PFOs.13,16 If a PFO is detected, evaluating for thrombophilia and deep venous thrombus (DVT) of the extremities may be warranted to assess for a potential source of paroxysmal emboli.6
Importantly, detecting a PFO in a patient with a cryptogenic stroke does not necessarily prove a causal relationship, given the frequency of the finding in the general population. Previous work has demonstrated that among patients with cryptogenic stroke who had a PFO, the PFO was probably the cause of stroke in less than 50%.16 PFOs in younger patients (≤60 years old), those with right-to-left shunting through the defect, and those with an associated atrial septal aneurysm are higher risk and are more likely to be the cause of stroke.14,15 PFOs in patients with known venous thrombus or in patients with a pure venous hypercoagulable state are more likely to have a causal relationship with stroke as well.16 In general, investigations to exclude alternative causes of stroke, particularly occult atrial fibrillation and underlying hypercoagulable states, are recommended prior to attributing cryptogenic stroke to the PFO.
Much debate has surrounded the treatment of PFOs in cryptogenic stroke over the last several years. Until relatively recently, antithrombotic therapy (typically with antiplatelet) was recommended over PFO closure after several studies showed no significant benefit of PFO closure over medical therapy alone.17,18 However, subsequent studies have shown benefit in stroke reduction with PFO closure in combination with antiplatelet in patients ≤60 years old and those with PFOs with high-risk features (i.e. intra-atrial shunt and atrial septal aneurysm).19 More recent data show persistent benefit of PFO closure despite age >60, suggesting that the high-risk features of the PFO should be considered over age when determining causal relationship of cryptogenic stroke and when optimizing treatment.20
As an aside, intrapulmonary shunts (i.e. pulmonary arteriovenous malformations or fistulas) are another cause of right-to-left shunting that may potentially cause ischaemic stroke and are typically detected by TTE or TOE during stroke evaluation.21,22 A few studies have shown a higher prevalence of these shunts in patients with cryptogenic strokes or TIAs.21,22 However, further work is needed in this area to better determine the clinical significance and association between intrapulmonary shunts and stroke.22
Aortic embolism
Aortic arch plaque is an important possible source of cryptogenic stroke. The risk of embolic events is highest with complex plaque. Complex plaque is defined as atherosclerotic plaque of ≥4 mm thickness and associated with ulcerations, mobile debris or superimposed thrombi.23 Aortic atherosclerosis is best assessed on TOE, but can also be seen on epi-aortic US and CT or MRI with or without angiography.24 In patients with ESUS undergoing TOE, 30% had aortic arch atherosclerosis and 8% had high-risk complex plaque.23 Proximal complex plaque was considered highest risk given its proximity to the main neck vessels.24 However, recent data suggesting that plaque is more distal in the descending aorta may also be suspicious as, during diastole, there is significant retrograde flow such that thrombi may reach the vessels of the neck and ultimately result in stroke.25,26 This is of particular interest because complex plaque is more frequently located in the distal aorta compared with the proximal aorta.27 In addition, it has been speculated that aortic atherosclerosis is more likely to cause strokes in the left hemisphere, as most atherosclerotic plaque is located distally to the innominate artery, which supplies circulation to the right-sided vasculature.27 However, this finding has not been consistent.23
The optimal treatment of aortic atherosclerotic disease in cryptogenic stroke is unclear. However, the current preference is a combination of antiplatelet medications and high-intensity statin therapy. Studies comparing anticoagulation with antiplatelet therapy have been inconclusive, and more research is needed in this area to define the best treatment.23,24,28
Sub-stenotic carotid disease
Traditionally, ischaemic strokes or TIAs were only attributed to carotid disease when the stenosis was greater than 50%.1 However, more recent data suggest that even carotid disease ≤50% can be pathologic and that the composition of the plaque determines its vulnerability and risk of causing TIA or stroke.29–31 Recent advancements in imaging have allowed for better characterization of plaque composition. Intraplaque haemorrhage, lipid-rich necrotic core and thinning or rupture of the fibrous cap of the atherosclerotic plaque are all associated with increased risk of embolic events.29–32 MRA with plaque composition protocol is the gold-standard technique, allowing for the most detailed assessment of plaque composition. However, CTA, positron emission tomography (PET) CT and ultrasonography with contrast enhancement can all be used to assess plaque morphology and characteristics, with varying sensitivity and specificity in detecting high-risk features.33
Despite this advancement, limitations still exist in the optimal treatment of non-stenotic carotid disease. The hope is that, with research, we will better know how to use this information to guide patient-specific treatment, i.e. medical therapy versus carotid intervention and, if intervention is warranted, which intervention is most appropriate. Further research is needed in this area.30,33,34
Carotid web
Though literature recognizing the entity is relatively sparce, carotid webs have been recently identified as an under-recognized cause of stroke in young patients without traditional stroke risk factors.35,36 A carotid web is defined radiographically as a posterolateral intraluminal filling defect of the proximal internal carotid artery, often described as a ‘shelf’ of tissue projecting into the lumen.35–37 Carotid webs can be appreciated with carotid US, though are often better seen on CTA or MRA, with the gold standard for detection being conventional angiogram.35,36 The composition of surgically resected carotid webs is consistent with fibrous proliferation, and thus carotid webs represent a form of focal atypical fibromuscular dysplasia rather than a variant of atherosclerotic disease.37 Most carotid webs are associated with only mild carotid stenosis. They may result in stroke due to blood stasis, thrombus formation and secondary embolization.37–40
Bilateral disease is not uncommon; almost 60% of patients with symptomatic disease will have asymptomatic contralateral disease as well.38,39 Symptomatic disease is seen most commonly in young women with a median age of 46 and of African American race.37,40
Despite an elevated rate of stroke recurrence with carotid webs,38,39 secondary stroke prevention is not well defined. Patients are often managed conservatively with antiplatelet therapy, but over half of patients have stroke recurrence within 1 year after the first event/stroke. Patients treated with anticoagulation have an even higher rate of recurrent stroke than those on antiplatelet therapy.37,40 Thus, carotid revascularization with either carotid endarterectomy or stenting has been proposed as an alternative treatment option with a much lower risk of stroke recurrence. Further work comparing long-term outcomes among different treatment options is needed to determine best management of this condition.36–38,41
Hypercoagulable state due to thrombophilia
The evaluation of cryptogenic stroke often includes testing for underlying thrombophilia (Table 1). Both inherited and acquired thrombophilia are associated with stroke, particularly in young patients and in those with prior personal or family history of thrombotic events.6
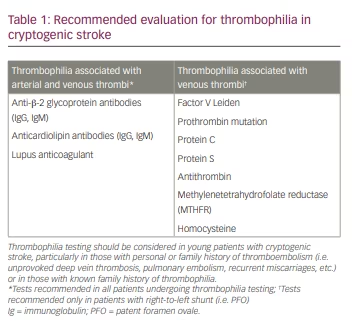
Inherited thrombophilia typically causes venous thromboembolism and may be implicated in cryptogenic stroke in the presence of cardiac and possibly pulmonary right-to-left shunts. Inherited thrombophilia includes deficiencies in protein C, protein S and antithrombin.42 Caution when interpreting abnormal results is warranted, as levels can be affected by illness, anticoagulant use and acute thrombosis. Often, repeat testing is needed to confirm. Genetic mutations such as factor V Leiden mutation, prothrombin G20210A gene mutation and elevated levels of factor VIII are also associated with stroke.43 Homozygosity or compound heterozygosity of factor V Leiden and prothrombin G20210A gene mutations lead to a considerably higher risk of thrombosis compared with heterozygosity for factor V Leiden mutation alone.42,43
Antiphospholipid syndrome (APLS) represents a relatively common cause of acquired thrombophilia that can result in both venous and arterial thrombosis, possibly causing cryptogenic stroke. Diagnosis of APLS requires a thromboembolic event and the presence of specific antiphospholipid antibodies (lupus anticoagulant, anti-β-2 glycoprotein-1 antibodies and/or anticardiolipin antibodies). Results of testing may be unreliable in the setting of certain illnesses or inflammatory states, recent thrombosis or anticoagulation. Thus, repeat testing 12 weeks following the initial testing is recommended to confirm the diagnosis.42,44
If stroke is attributed to an underlying thrombophilia, long-term anticoagulation therapy is generally recommended for secondary stroke prevention. Consultation with haematology is recommended.43
Malignancy
Malignancy is associated with a pro-inflammatory and hypercoagulable state and can result in ischaemic stroke through various mechanisms.45,46 Though uncommon, ischaemic stroke can be the first presentation of cancer. Overall, the incidence of cancer detected after ischaemic stroke is low but is higher in cases of cryptogenic stroke. Though testing for malignancy is not necessary for all patients with cryptogenic stroke, certain historical or clinical features, such as older age, smoking history, the presence of constitutional symptoms and elevated inflammatory or pro-thrombotic makers, should raise concern and prompt further evaluation.47
In general, outcomes are typically worse for patients with cancer who have a stroke. This may be, in part, due to debility related to the initial stroke and recurrent strokes.47,48 Alongside treating the underlying malignancy, anticoagulation with low-molecular-weight heparin has been best studied and is recommended as first-line therapy for secondary stroke prevention in cancer-related stroke. Warfarin has also been used with favourable results. The more convenient direct oral anticoagulants, such as apixaban, are becoming more frequently used but with mixed results.46,49,50
Genetic syndromes associated with stroke
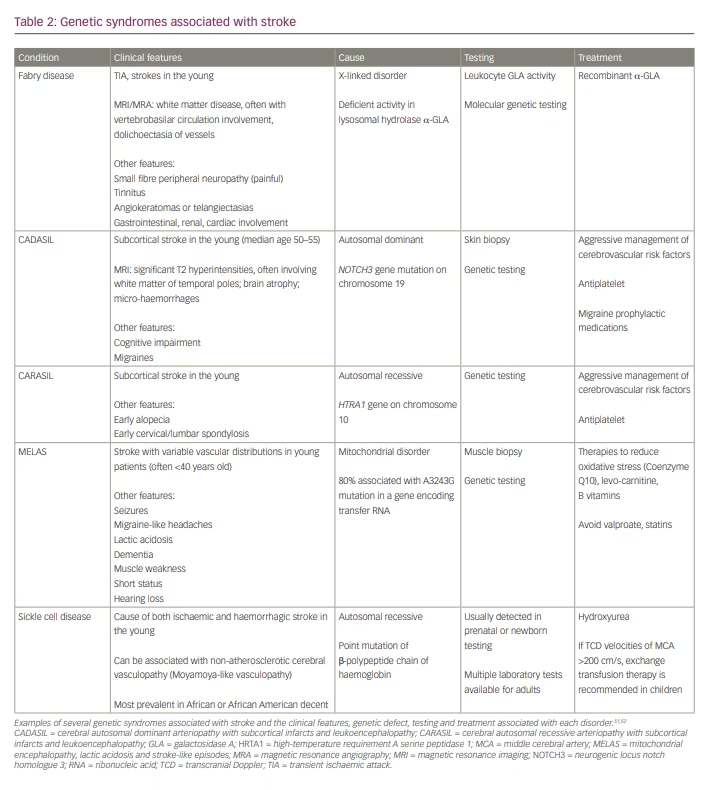
Inherited stroke syndromes should be considered in young patients with a family history of stroke in the young, and in those with recognizable clinical phenotypes of specific genetic syndromes (Table 2). When clinical suspicion is high for an individual syndrome, genetic testing should be considered, as an accurate diagnosis may have therapeutic implications and allow for familial genetic counselling.51,52
Non-atherosclerotic vasculopathy
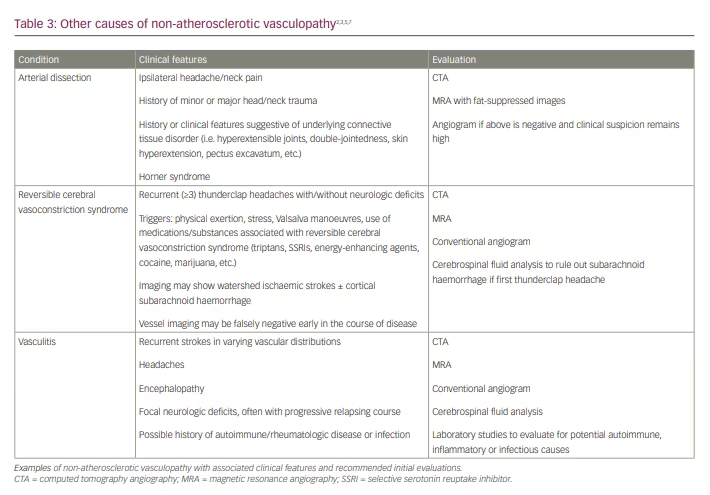
Other, non-atherosclerotic vasculopathies, such as arterial dissection, reversible cerebral vasoconstriction syndromes and vasculitis are among potential causes for cryptogenic stroke. Typically, patients will present with features that raise suspicion for a specific condition, and diagnostic evaluation and treatment should be tailored to the individual (Table 3).2,3,5,7
Summary and conclusions
Cryptogenic stroke is common and most often occurs in younger patients with fewer traditional risk factors for stroke.4–5 Given the higher risk of recurrent stroke, a thorough evaluation for potential mechanisms is warranted.5,7 All patients should undergo a standard comprehensive evaluation to assess for the most common mechanisms of stroke. Additional studies beyond the initial evaluation should be tailored to the patient’s clinical history and risk factors as much as possible;6 evaluations for cryptogenic stroke are often costly if not covered by insurance, are potentially unavailable in less resource-rich locations and are of relatively low yield. The best treatment for secondary stroke prevention is dependent on the mechanism of stroke, and risk-factor modification and antiplatelet therapy are often recommended in the absence of definitive cause.1,49–50














