In clinical practice, the definition of penumbra is largely based on neuroimaging and indicates potentially salvageable tissue. Perfusion imaging has been useful in identifying this tissue when obeying ‘the mismatch concept’. The mismatch concept is a surrogate marker for salvageable brain tissue and refers to a lesion volume difference (mismatch) between the perfusion deficit (the total volume of brain tissue that is hypoperfused) and the ischaemic core. This mismatch serves as both a diagnostic and management decision-making tool in ischaemic stroke.1
In 2012, the Diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) 2 trial defined the mismatch criteria for endovascular thrombectomy (EVT)-eligible patients using a magnetic resonance imaging (MRI) profile: mismatch ratio ≥1.8, penumbra ≥15 mL, diffusion-weighted imaging volume <70 mL and a Tmax >10 s for volume <100 mL.2 These criteria are based on the expectation of a greater benefit in patients with a larger reversible perfusion deficit. However, of the 92 patients who underwent EVT in the trial, the minimum National Institutes of Health Stroke Scale (NIHSS) score was 10.2 Furthermore, in 2015, when the New England Journal of Medicine released the results of five separate landmark trials (MR CLEAN, EXTEND-IA, ESCAPE, SWIFT PRIME and REVASCAT) for further development of guidelines for EVT in large-vessel occlusion (LVO), only MR CLEAN and EXTEND-IA included very limited participants with low NIHSS scores.3,4
Thus, this retrospective single-centre study was performed to evaluate neuroimaging indicators to pursue reperfusion in patients with acute ischaemic stroke (AIS), LVO and low NIHSS scores. Our study included patients with an NIHSS score of <8 to cover boundary cases, where an NIHSS score is felt to be close to 6, and to help decide whether there is an indication for pursuing EVT. This is the first study to report perfusion findings in strokes with NIHSS scores of <8 and LVO while also including the implications of these findings on decision-making to pursue EVT and outcomes.
Methods
Patients and data collection
All consecutive patients with anterior circulation LVO and NIHSS scores of <8 who were admitted to the inpatient stroke service at Augusta University Medical Center from 1 January 2019 to 31 December 2019 were included in the study. Inclusion criteria were patients with an NIHSS score of <8 and LVO of the M1 and M2 branches of the middle cerebral artery, anterior cerebral artery and internal carotid artery. Three treatment groups were formed: medical, urgent and rescue. The urgent group included patients who underwent EVT in <6 hours from presentation. The rescue group included patients who underwent EVT >6 hours from presentation with increasing NIHSS; this included patients who presented >6 hours from symptom onset and underwent urgent EVT. The maximum period that EVT was allowed in the rescue group was up to 24 hours from presentation of symptom onset. Last known door-to-groin puncture/recanalization times for the urgent and rescue groups were not recorded in this study. Patients in all groups received intravenous tissue plasminogen activator (tPA) according to institutional guidelines. The therapy decision was made by the vascular neurologist and neurointerventionalist on-call, based on clinical course and perfusion imaging. The decision to pursue perfusion imaging was determined by clinical history and neurological examination.
Data on and history of age, sex, hypertension, diabetes mellitus, hyperlipidaemia, tobacco use, coronary artery disease, atrial fibrillation, anticoagulation, side of stroke, site of occlusion, NIHSS on arrival, tPA administration, collateral score (obtained during invasive angiogram), NIHSS score at discharge, thrombolysis in cerebral infarction (TICI) score, modified Rankin Scale (mRS) score at discharge, and mRS score at the 90-day follow-up in-person clinic visit were collected. Computed tomography (CT) perfusion metrics acquired via RAPID software (iSchemaView, Menlo Park, CA, USA), including cerebral blood flow (CBF) <30%, Tmax >6 s (volume in mL), mismatch volume and mismatch ratio, were also collected.
Statistical analysis
All statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and statistical significance was assessed using an alpha level of 0.05.
Two different definitions of change in NIHSS score from arrival to discharge were created, each calculated as discharge minus arrival. The first (NIHSS shift 1+) was defined as an increase of 1 or more, indicating worsening of the NIHSS score, a 0 value indicated no change and a decrease of 1 or more indicated a better NIHSS score at discharge. The second (NIHSS shift 2+) was defined as an increase of 2 or more, indicating worsening of the NIHSS score, a value between -1 and +1 indicated no change and a decrease of 2 or more indicated a better NIHSS score at discharge.
The mRS score at discharge was categorized as better for values of 2 or less and worse for values of 3 or more. TICI scores of 0, 1 or 2a were categorized as unsuccessful reperfusion, and 2b or 3 were categorized as successful reperfusion.
Descriptive statistics were determined for all variables overall and by intervention group (urgent, rescue or medical). To examine preliminary associations between intervention and the categorical and continuous demographic, clinical history, arrival, imaging, discharge and follow-up variables, chi-squared tests or one-way analysis of variance (ANOVA) models were used. If the assumptions to the chi-squared test were violated, a Fisher’s exact test was used. For the ANOVA models, a Tukey–Kramer multiple comparison test was used to examine post hoc pairwise differences between intervention groups.
Results
We reviewed 49 patients presenting with AIS, LVO and an NIHSS score of <8 who underwent either medical therapy (n=27), or rescue (n=10) or urgent (n=12) thrombectomy. The average age of patients was 62.2 (standard deviation 13.7) years, and 51.0% were male (Table 1). Overall, 79.6% had hypertension, 16.3% had diabetes, 24.5% had hyperlipidaemia, 38.8% used tobacco, 18.4% had coronary artery disease, 20.4% had atrial fibrillation and only 4.1% were on anti-coagulation therapy. The majority of patients had a right-side stroke, and the occlusion site with the highest frequency of occurrence was M1. The mean arrival NIHSS score was 3.9, and 49.0% of patients were administered tPA. There were no differences in demographic variables between groups (Table 1). In-hospital mortality was 6.0%, with a total mortality at 90-day follow-up of 13.0%.
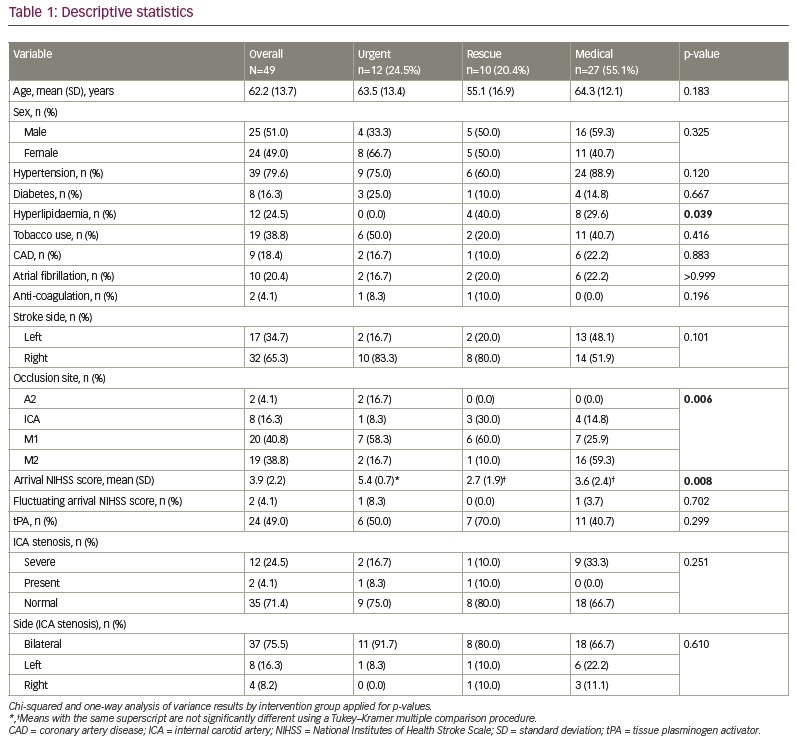
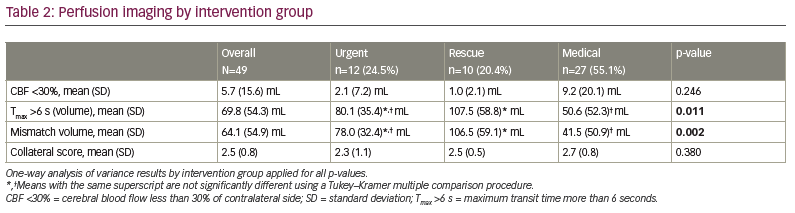
Intervention group differences were seen for hyperlipidaemia, occlusion site, arrival NIHSS score, Tmax >6 s, mismatch volume, NIHSS shift 1+ and NIHSS shift 2+ (Tables 1, 2 and 3). Patients in the rescue or medical groups had higher percentages of hyperlipidaemia than in the urgent group. The distribution of occlusion site was different, with those in the urgent or rescue groups having the highest percentage of strokes in M1, and those in the medical group having the highest percentage in M2. The arrival NIHSS score was significantly higher in the urgent group than in the rescue group (Tukey–Kramer adjusted p=0.009) or medical group (Tukey–Kramer adjusted p=0.033). The Tmax >6 s was significantly greater in the rescue group compared with the medical group (p=0.011) but not the urgent group (p=0.420), and the urgent and medical groups did not have a significantly different Tmax >6 s (p=0.220). The mismatch volume was greater in the rescue group compared with the medical group (p=0.002) but not the urgent group (p=0.370), and the urgent group did not have significantly different mean mismatch volume compared with the medical group (p=0.09). The urgent group displayed TICI scores of 2b/3 in 100% of patients, whereas the rescue group displayed TICI scores of 2b/3 in 80% and 1/2a in 20% (p=0.076). The perfusion core (CBF <30%) was not different between the groups (2.1 cm3, 1.0 cm3 and 9.2 cm3, for urgent, rescue and medical groups, respectively).
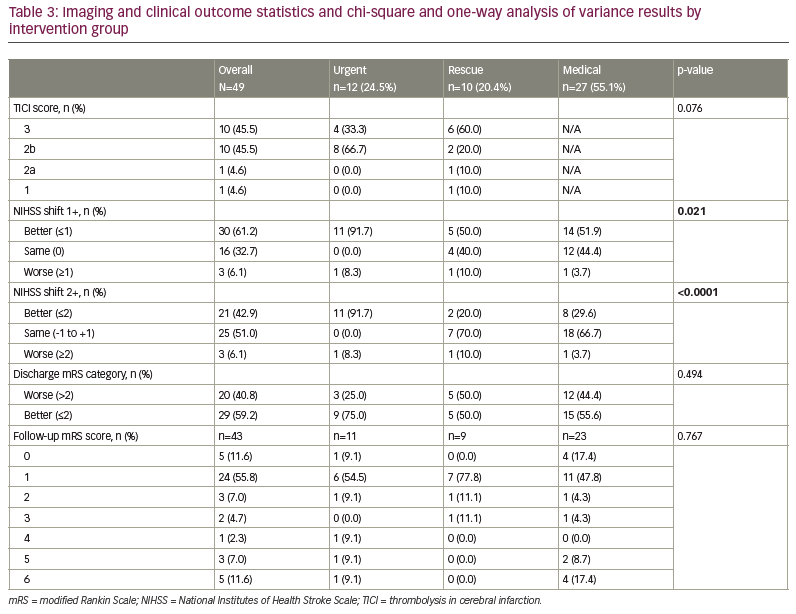
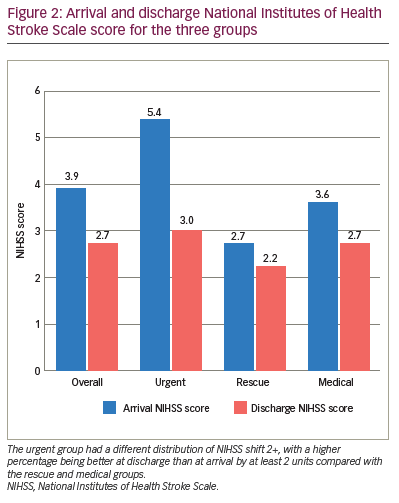
Patients in the urgent group had a different distribution of NIHSS shift 1+, with a higher percentage being better at discharge than at arrival by at least 1 unit (91.7%) compared with the rescue (50.0%) and medical (51.9%) groups (Table 3). Those in the urgent group had a different distribution of NIHSS shift 2+, with a higher percentage being better at discharge than at arrival by at least 2 units (91.7%) compared with the rescue (20.0%) and medical (29.6%) groups. The majority of those in the rescue (70.0%) and medical (66.7%) groups had a similar NIHSS score (change between -1 and 1) at discharge compared with arrival. The arrival NIHSS score was significantly higher in the urgent group (5.4) compared with the rescue (2.7) and medical groups (3.6) (Figure 2).
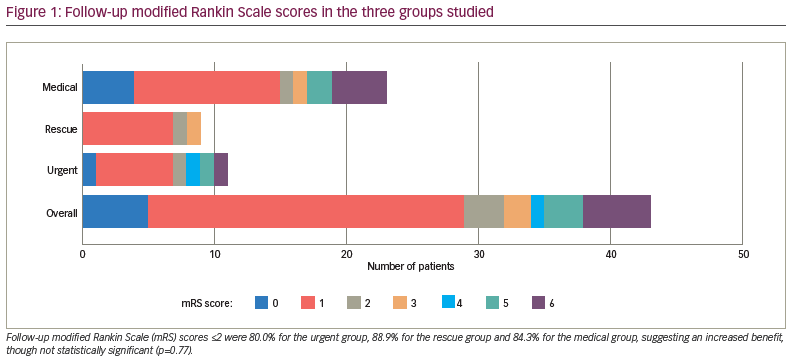
Change in mRS score of ≤2 on discharge occurred in 75.0% of patients in the urgent group, 50.0% in the rescue group and 55.6% in the medical group, suggesting an increased benefit but not a statistically significant one (p=0.250). Follow-up mRS scores ≤2 occurred in 80.0% of the urgent group, 88.9% of the rescue group and 84.3% of the medical group (p=0.770) (Figure 1). The urgent group had a different distribution of NIHSS shift 2+, with a higher percentage being better at discharge than at arrival by at least 2 units compared with the rescue and medical groups (Figure 2). One potential confounder was a difference in reperfusion between urgent thrombectomy with TICI of 2b/3 in 100% of patients. The rescue group displayed TICI reperfusion scores of 2b/3 in 80% and 1/2a in 20% of patients, but this difference was not statistically significant (p=0.076).
Discussion
Literature regarding low NIHSS scores and use of EVT with improved outcomes is both increasing and evolving. Our study helps to reinforce the findings in existing publications, many of which are included in a large meta-analysis performed by McCarthy et al.5 This meta-analysis reviewed over 24 quantitative and six qualitative studies investigating the efficacy of EVT in patients with AIS, LVO and low NIHSS scores. Definitions of low NIHSS scores in these multiple studies included scores ≤5, ≤7 and ≤8. Our study further expands these findings by including CT perfusion deficits (particularly mismatch volume and Tmax) as a driver for EVT in AIS with low NIHSS scores (NIHSS score <8); few previous studies have investigated this. A case report by Nguyen et al. highlighted the importance of MRI perfusion as a decision-making tool to proceed with EVT in a 55-year-old male patient who had a transient ischaemic attack. He had an NIHSS score of 0 but was found to have a proximal M1 occlusion with an MRI perfusion scan revealing a large ischaemic penumbra of 70 cm3 with small acute lesions on the left hemisphere with a core volume of 0 mL. Although this was a single case study, it does illustrate that EVT, even in patients with an NIHSS score of 0, could be an effective and safe treatment.6
Furthermore, two large randomized controlled trials studying the efficacy of EVT in patients with AIS, LVO and a low NIHSS score are currently being conducted: Endovascular therapy for low NIHSS ischemic strokes (ENDOLOW)7 and Minor stroke therapy evaluation (MOSTE)8 studies. ENDOLOW is a trial based in North America that will enrol 200 patients presenting within 8 hours of symptom onset, with randomization to either EVT with EmboTrap II (CERENOVUS, Irvine, CA, USA) or medical management. MOSTE is a trial based in Europe that will enrol 824 patients presenting within 24 hours of symptom onset with randomization to EVT or medical management. These trials highlight the importance of this subject and validation of other studies contributing to it.
The penumbra was classically defined as the hypoperfused tissue surrounding the ischaemic core in which blood flow is too low to maintain electrical activity but sufficient to preserve ion channels.1 This tissue is prone to becoming an extension of the ischaemic core, as well as liable to harmful excitotoxicity, oxidative stress and inflammation if not reperfused.1 In clinical use, the penumbra is determined by subtracting the ischaemic core from the perfusion deficit.1 The mismatch concept used in perfusion imaging serves as an indicator for EVT in higher NIHSS scores, and our results suggest that it may also serve as an indicator for EVT in those with lower scores (NIHSS score <8) with LVO. Our study suggests that perfusion mismatch, higher NIHSS scores and timing should be considered when determining EVT management in patients with lower NIHSS scores.
The RAPID-AI CT perfusion software, which is used at our institution, has proven to be successful in identifying perfusion mismatch and in aiding management in AIS.9 We found that the perfusion core (CBF <30%) was similar between the groups in our study (2.1 cm3, 1.0 cm3 and 9.2 cm3 for urgent, rescue and medical groups, respectively). However, the perfusion penumbra (Tmax >6 s [volume]) and mismatch (Tmax minus CBF) were significantly larger for the urgent and rescue groups: 80.1 cm3 and 107.5 cm3, respectively, compared with 50.6 cm3 for the medical group (p=0.0112) for Tmax, and 78.0 cm3 and 106.5 cm3, respectively, compared with 41.5 cm3 for the medical group (p=0.0022) for mismatch. Follow-up mRS scores ≤2 suggested an increased benefit for the urgent group, though this was not statistically significant (Figure 1). These results suggest that intervention in larger penumbra volumes may have better outcomes, as larger brain volumes are salvaged. This conclusion is in line with previous studies comparing endovascular versus medical therapy in AIS and higher NIHSS scores with LVO.10
The decision to pursue EVT may also be driven by a higher NIHSS score, combined with non-contrast CT showing a small core, and CT angiogram to identify clinical radiological mismatch for NIHSS scores of ≥6 and LVO as in previous studies.11 In our study, the arrival NIHSS score was significantly higher in the urgent group compared with the rescue and medical groups (Figure 2). Although immediate intervention led to the best outcomes, improvement was also noted in the rescue group. This is similar to a finding in a previous study indicating that outcomes are still better with thrombectomy plus standard care versus standard care alone up to 24 hours from symptom onset for patients with higher NIHSS scores.10 In our study, the medical management group with no worsening had a mismatch of 41.5 cm3 versus 106.5 cm3 for the rescue group. The mismatch volume that predicts clinical worsening after a low NIHSS score presentation is likely to fall between these two values and should be investigated further.
One potential confounder was a difference in reperfusion between urgent thrombectomy with TICI of 2b/3 in 100% of patients. The rescue thrombectomy group displayed TICI reperfusion scores of 2b/3 in 80% and 1/2a in 20%, but this difference was not statistically significant (p=0.076). However, it may be that a shorter time to intervention led to higher reperfusion rates and improved outcomes. Other studies have demonstrated higher quality reperfusion with decreased onset-to-treatment time in anterior circulation LVOs.12,13 This might be explained by the physiology of thrombus formation and the coagulation cascade.
With time, the composition of the thrombus evolves. The coagulation cascade has more time to become activated resulting in haemostasis via formation of a fibrin mesh. A thrombus with a higher fibrin concentration is difficult to remove with a stent retriever, preventing optimal reperfusion.14 Finding clear markers, including time to intervention, for proceeding to EVT in patients with LVO and low NIHSS scores may further improve outcomes.
Overall, the combination of NIHSS score, non-contrast CT, blood vessel and larger perfusion deficits for EVT candidate selection may be applicable for patients with lower NIHSS scores.
Limitations of our study include the single-centre and non-randomized design. Other limitations include the lack of analysis of early neurological deterioration, small sample groups and not labelling the lower NIHSS scores as disabling or non-disabling. Ethnic groups represented were Caucasian and African American. Criteria for reperfusion also relied on clinical acumen through the combination of clinical and perfusion imaging.
Conclusion
Larger perfusion deficits on CT perfusion imaging may serve as an indicator for EVT candidacy in AIS with LVO and a NIHSS score of <8. The biggest driver for urgent reperfusion was a larger penumbra, which appeared to show a better outcome, as seen by improvement in NIHSS score at discharge, without any difference in 3-month outcomes graded by mRS scores. Indications for, and results from, thrombectomy in LVO presenting with NIHSS score <8 should be and are being investigated in larger randomized controlled clinical trials.














