Introduction
Safinamide is an innovative therapeutic option in Parkinson’s disease (PD), with a unique dual mode of action that goes beyond dopaminergic pathways.1 Its mechanisms of action include monoamine oxidase-B (MAO-B) and dopamine reuptake inhibition, activity-dependent sodium-channel inhibition, and inhibition of glutamate release.2
In pivotal clinical trials, safinamide used as add-on to levodopa, demonstrated efficacy in improving motor symptoms and motor fluctuations in patients with mid- to late-stage PD, as indicated by significant improvements in Unified Parkinson’s Disease Rating Scale (UPDRS) III scores, a reduction in OFF time, and an improvement in ON time without troublesome dyskinesia.3,4 Safinamide was also effective for improving non-motor symptoms such as pain and mood.5,6
During this symposium, which was held during the 3rd Congress of the European Academy of Neurology (EAN), the expert faculty explored the potential value of safinamide as a unique PD therapy, through a series of patient case presentations.
Patients with mid- to late-stage PD who might potentially benefit from the addition of safinamide to their current regimen include those with levodopa-induced motor complications and patients whose motor and/or non-motor symptoms are not adequately controlled with levodopa and other PD therapies.
Patients who desire better control of motor and non-motor symptoms
Cases presented by Jaime Kulisevsky
Movement Disorders Unit, Neurology Department, Sant Pau Hospital, Autonomous University of Barcelona, CIBERNED and Universitat Oberta de Catalunya, Barcelona, Spain
Motor complications – namely, fluctuations and dyskinesias – are a frequent complication of levodopa treatment in patients with PD. In one study of levodopa-treated patients, 28% were experiencing treatment-induced dyskinesias and 40% reported motor fluctuations. The prevalence of both types of motor complications increased with the duration of PD. Interestingly, motor fluctuations were most strongly related to disease duration and dose of levodopa whereas dyskinesias were best predicted by duration of treatment.7
Patients with PD may also experience fluctuations in non-motor symptoms, which can be as distressing as motor fluctuations. Examples of non-motor symptoms include anxiety, shortness of breath, diaphoresis, paresthesias, depression, and pain. These symptoms are frequently not recognised by the patients themselves or their physicians, despite being highly prevalent.
In one study using a comprehensive screening questionnaire, the 30-item NMSQuest, PD patients reported an average of 10 different non-motor symptoms each. These symptoms were highly prevalent across all disease stages and the number of symptoms increased significantly with advancing disease and duration of disease. Just 2.5% of PD patients were completely free from non-motor symptoms. Many of the symptoms identified with NMSQuest – such as diplopia, dribbling, apathy, taste and smell problems – had not been previously disclosed to health professionals.8
Case 1
History and presenting complaint
The patient is a 63-year-old woman with PD but otherwise healthy, who has been receiving levodopa/carbidopa 300 mg/day for some time. She has no motor fluctuations but complains of transient “confusion” after each levodopa dose; her daughter says she has little energy and is losing her ability to cook.
Treatment plan and outcome
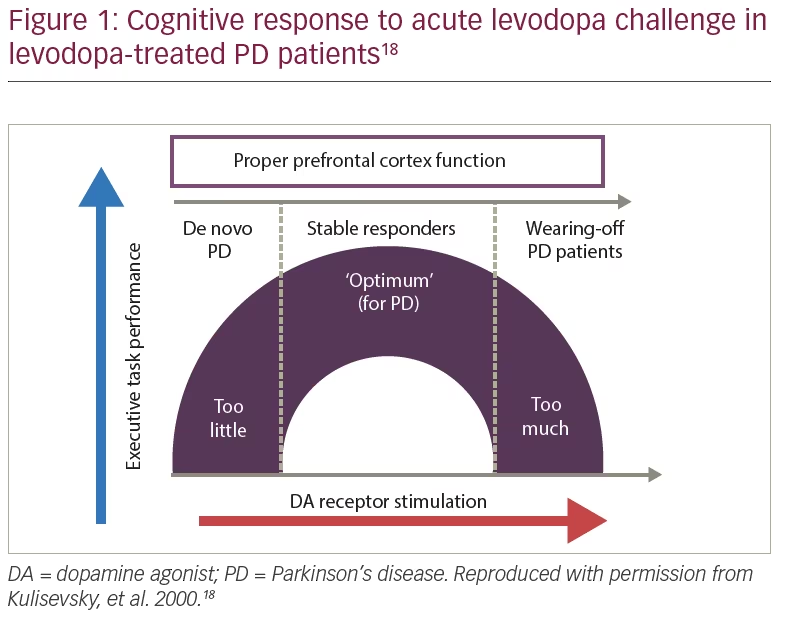
The patient was started on safinamide 100 mg/day and levodopa was reduced to 225 mg/day. The “overdose” effect, which is more common in patients with wearing-off, disappeared (Figure 1); she showed no worsening in tremor or other parkinsonian signs, and no dyskinesias.
Case 2
History and presenting complaint
The patient is a 55-year-old man with PD of 5 years’ duration, treated with levodopa/carbidopa/entacapone 100/25/200 mg four times daily, plus pramipexole ER 1.05 mg once daily. He complains of motor fluctuations (tremor, loss of finger dexterity, walking difficulties) for the past 1 year which are worse on waking or if a dose is missed. He denies dyskinesias but his wife has observed grimacing after the midday levodopa dose. The patient also experiences anxiety and bradyphrenia 30 minutes before each levodopa dose, mainly in the afternoon.
Treatment plan and outcome
The patient was started on safinamide 100 mg/day. After 1 month, his motor/non-motor fluctuations had disappeared. He experienced a slight increase in facial dyskinesias, which resolved on reduction of his levodopa dose to 100/25/200 mg four times daily.
Case 3
History and presenting complaint
The patient is a 78-year-old woman with PD of 13 years’ duration, being treated with levodopa/carbidopa 200/50 mg four times daily, quetiapine 25 mg once daily, and citalopram 20 mg once daily. She reports slight early-morning wearing-off, some freezing episodes and gait disturbance, low mood, visual hallucinations in the late afternoon, and mild cognitive impairment. The main complaints of the patient and her family are repeated episodes of anxiety, diaphoresis and shortness of breath.
Treatment plan and outcome
The patient was started on safinamide 100 mg/day. One month later her anxiety symptoms had disappeared, with no side effects or increase in hallucinations, and the positive response persisted at 8-month follow-up.
It is important to identify the pattern of occurrence of anxiety disorder in PD. Anxiety may be related to off-periods, sometimes arising in parallel to motor fluctuations; it may be unrelated to motor fluctuations, being either a comorbid symptom or a presenting symptom of PD; it can predate motor symptoms by many years; or anxiety can be part of the PD premorbid personality, along with traits such as less novelty-seeking and inflexible character.
In a study assessing anxiety fluctuations in PD, patients were treated with two levodopa formulations with different time-to-peak plasma levels. Analysis of dose-response curves showed that motor status strongly correlated with levodopa plasma levels and anxiety, particularly in patients with wearing-off taking immediate-release levodopa. The findings suggest that recurrent motor impairment behaves as a stressor and is an important factor explaining anxiety swings in mid- to late-stage patients with PD.9
Case 4
History and presenting complaint
The patient is a 44-year-old man with PD of 8 years’ duration, who reports mild wearing-off, stabilised with four doses of Stalevo® 150 (levodopa 150, carbidopa 37.5 and entacapone 200 mg; Novartis, Basel, Switzerland). He complains of rapid onset of intense pain in left arm and hand, occasional dystonic contracture of left foot, and hobbyism with ropinirole. The symptoms begin 45 minutes before each dose of Stalevo® and subside from 15 minutes post-dose. Previously, increasing the Stalevo dose resulted in dyskinesias and treatment with rasagiline 1 mg/day was not effective.
Treatment plan and outcome
The patient was started on safinamide 50 mg, increasing to 100 mg/day. This resulted in a consistent improvement of pain; occasionally he still experienced pain of milder severity 15 minutes before each dose of Stalevo®.
In conclusion, although various treatment options were available to these four patients, all were treated with safinamide 100 mg/day and all patients showed clinically relevant improvements in their non-motor symptoms. Furthermore, safinamide was well tolerated in all patients.
Patients who are poorly controlled on multiple pharmacological therapies
Case presented by Alain Kaelin
Neurocenter of Southern Switzerland, Manno, Switzerland
The patient* is a 78-year-old man with PD of 5 years’ duration. He presented with subjectively very disturbing symptoms: morning OFF with freezing, postural instability even when ON, a slight intermittent tremor, and non-motor symptoms (dizziness, incontinence). He did not have dyskinesia.
Significantly, in his medical history he suffered a subdural haematoma 17 years ago, which might explain some of his executive function deficits. He had undergone surgery for colon cancer and a transurethral resection of the prostate 11 years ago. On physical examination (partial ON) he had a UPDRS III score of 30, no tremor, right-sided bradykinesia, and postural instability on the pull test.
At the insistence of the patient and his family, he was admitted to the neurology unit for assessment. His treatment was Sinemet® CR (carbidopa and levodopa, extended release tablet; Merck Sharp & Dohme Corp., Whitehouse Station, NJ, US) 50/200 mg four times daily. A 7-day patient diary revealed lots of ON time but early-morning wearing-off and some tremor (Figure 2a). The objective findings were therefore less severe than the patient’s subjective complaints.
There are many treatment possibilities for patients with PD who are experiencing levodopa-associated motor fluctuations and dyskinesia. This was illustrated in a survey of neurologists, who cited 13 different treatment strategies for such a patient. The most popular choices involved lowering (or fractionating) the levodopa dose, sometimes with the addition of a second therapy.10 This approach is supported by the STRIDE-PD study, which showed that the risk of developing dyskinesia or wearing-off was closely related to levodopa dose and increased markedly at daily doses above 400 mg.11
For the patient described above, two treatment options – deep brain stimulation (DBS) or an increase/change of dopamine agonist – can be ruled out. DBS is not suitable for patients with previous brain injury and cancer surgery, while a change of dopamine agonist is not appropriate since he is already taking pramiprexole 3 mg with good ON during the day.

There are two other options, the first of which would be to increase the overall levodopa dose. While this is simple and has few side effects, it also increases the risk of dyskinesia. The second option would be to add-on another therapy, such as a catechol-O-methyltransferase (COMT) or MAO-B inhibitor. These also have few side effects and do not require a change in the levodopa dose.
The decision for this patient was to add safinamide, starting at 50 mg and increasing to 100 mg/day. He was also switched to fast-acting levodopa as rescue therapy in the morning (0.5–1.0 x 125 mg soluble levodopa/benserazide). The patient diary showed a clear improvement, with a complete absence of OFF time (Figure 2b).
Safinamide’s beneficial effect on OFF time has been demonstrated in randomised controlled trials. In study 018, OFF time (a secondary efficacy endpoint) decreased significantly between baseline and Week 78 in patients treated with safinamide (least-squares mean difference versus placebo: -0.62 hours with safinamide 50 mg and -0.75 hours with safinamide 100 mg).12 Although OFF time was reduced by less than 1 hour per day, such an effect would be clinically relevant in patients such as the case described above.
This case study illustrates how diaries can reveal how the patient’s status fluctuates in relation to time of day and levodopa dose. For older patients with multiple comorbidities, levodopa should be given at the lowest possible dose, plus adjunctive therapies to optimise motor control if required. Safinamide has demonstrated long-term beneficial effects on OFF time as well as control of motor fluctuations. Safinamide’s unique mechanism of action, with modulation of both dopamine and glutamate systems, may maximise motor symptom control in PD without worsening motor complications.
*Written informed consent was not obtained from the patient case included in this report; no identifying information or images were used in the publication of this paper.
Patients with motor complications who need an add-on to levodopa
Case presented by Fabrizio Stocchi
Department of Neurology, University and Institute for Research and Medical Care (IRCCS), San Raffaele, Rome, Italy
The case is a 68-year-old man with PD of 8 years’ duration. His initial symptoms were intermittent tremor of his right hand plus a subjective feeling of poor coordination of his right leg when walking. He was started on ropinirole 12 mg daily, which resulted in marked and sustained relief from his symptoms. After approximately 1 year he began to experience re-emergence of tremor and gait difficulties with some general slowness. Ropinirole was increased to 20 mg daily, again with good effects. After another year he began to notice re-emergence of symptoms so levodopa, 100 mg four times daily, was added. His motor fluctuations partially resolved for 3 months then re-emerged.
At next presentation, his medication was Sinemet 250/25 mg, half a tablet four times daily, ropinirole prolonged release 20 mg, and amitriptyline 10 mg. He had normal cognitive function, no psychiatric symptoms, mild depression, and nocturnal akinesia. As the patient was complaining of tremor and slowness, the neurologist judged the patient to be undertreated, and increased levodopa to 150 mg four times daily. However, 12 months later the patient continued to complain of tremor and difficulty walking, so levodopa was increased to 200 mg four times daily.
On assessment, the patient was initially suffering tremor and difficulty walking, which disappeared during the consultation. This suggested that he was experiencing wearing-off and peak-dose dyskinesia, which had not previously been recognised. Treatment options for this patient include: increasing the ropinirole dose, switching to another dopamine agonist, adding a MAO-B inhibitor or entacapone, increasing the levodopa dose, considering tolcapone or deep brain surgery, adding levodopa oral solution or duodenal infusion, or starting subcutaneous apomorphine (continuous infusion or intermittent pen injector).


For patients with wearing-off and peak-dose dyskinesia, it is important to determine whether the levodopa dose is correct. In the current case, it is likely that the patient’s dose is too high. Importantly, with traditional levodopa, plasma levels of the drug rise and fall with each dose, and this pulsatility is still present at higher levodopa doses (Figure 3).13 However, increasing the dose leads to variable control, with peak-dose dyskinesias and wearing-off (Figure 4).14
In order to improve this patient’s symptom control, his levodopa dose should be reviewed and an adjunctive therapy started. Entacapone and tolcapone are possible adjunctive therapies to improve motor control but can worsen dyskinesia, so are not suitable in this case. Levodopa was reduced to 150 mg four times daily (at 4-hour intervals) and safinamide 100 mg was added. At follow-up, the patient reported an improvement in wearing-off without any worsening of dyskinesia.
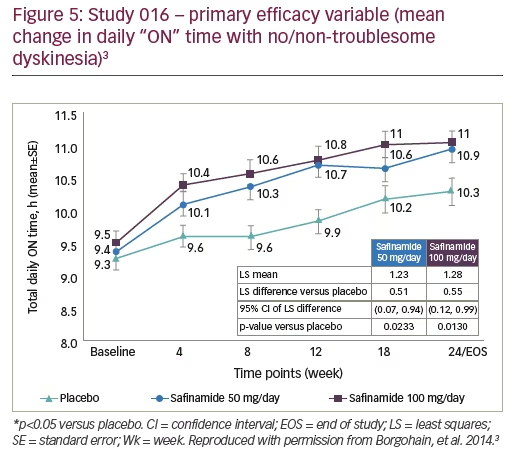
The efficacy of safinamide in improving daily ON time was demonstrated in the phase III 016 study and its long-term extension study, 018 (Figures 5 and 6). At 6 months, patients treated with safinamide experienced a significant increase in ON time with no/non-troublesome dyskinesia (least-squares mean difference versus placebo: +0.51 hours with safinamide 50 mg and +0.55 with safinamide 100 mg).3 This improvement was maintained over 2 years, indicating a long-term clinical effect.12 An interesting observation when analysing the mean change in daily “ON” time with no/non-troublesome dyskinesia, in the 018 study, is the magnitude of the placebo effect; although this decreased over time, it was still present and significant at 2 years (Figure 6).
Safinamide has demonstrated efficacy in reducing dyskinesias. The primary endpoint of the long-term 018 study was the change in mean Dyskinesia Rating Scale (DRS) score during ON time between baseline (i.e., randomisation in the 016 study) and 2 years. For the overall study population (with or without dyskinesias), DRS scores decreased by 31% and 27%, with safinamide 50 and 100 mg/day, respectively, versus 3% with placebo, a non-significant difference (Figure 7).12


Around 25% of the overall 018 study population had moderate-severe dyskinesia at baseline (DRS score >4). In a post-hoc analysis involving this subgroup, safinamide 100 mg resulted in a significant improvement in DRS scores at 2 years (Figure 7).12 The favourable effect of safinamide on dyskinesia in the long term may be explained by inhibition of state- and use-dependent Na+ channels and stimulated glutamate release.15
With regards to safinamide dosing, both 50 mg and 100 mg doses were significantly effective in improving ON time, OFF time, and UPDRS III scores, in the 018 study.12 For some secondary efficacy variables, however – UPDRS II, UPDRS IV, and Parkinson’s Disease Questionnaire (PDQ)-39 total score (a measure of quality of life) – only the 100 mg dose was significantly more effective than placebo.3 Similarly, only safinamide 100 mg had a significant benefit on PDQ-39 subscale scores (activities of daily living, emotional wellbeing, communication and bodily discomfort).12
These dose-related differences suggest that quality of life in PD patients is determined not only by motor symptoms but by other factors. The pharmacology of safinamide may help explain these findings. The 50 mg dose is sufficient to block the MAO-B enzyme, but only the 100 mg dose also has effects on glutamate and other non-dopaminergic pathways that may be particularly relevant to non-motor symptoms and quality of life.
In conclusion, this case teaches us that wearing-off is not always recognised by patients or doctors. One should remember that the patient’s own account of their symptoms is by definition subjective, and their description can sometimes lead to the wrong conclusions. Physicians must be meticulous in documenting symptoms and response to treatment in order to identify the presence of fluctuations. Furthermore, in patients with wearing-off, increasing the levodopa dose will have no benefit. Finally, safinamide has proven efficacy in improving wearing-off without increasing dyskinesia.
A patient on safinamide with good symptom control who worries about future decline
Case presented by Heinz Reichmann
Technische Universitaet Dresden, Germany
The case is a 55-year-old neurosurgeon who initially noticed a slight tremor in his left arm, which was worse when he was under stress, together with micrographia and slight loss of dexterity in the left hand. His family history was normal and he had no pre-existing medical conditions other than migraines. He also had some premotor signs (loss of smell, some depressive moments).
Neurological examination revealed hypomimia, a left-sided resting tremor, and minor postural tremor. Dexterity of his left arm was slightly impaired and he had micrographia even when writing with his right hand. Postural stability, cognition and mood were normal.
To confirm the diagnosis the patient underwent quantitative tremor analyses, which revealed a left-sided parkinsonian tremor as well as a slight essential tremor – findings typical for PD (Figure 8). He also underwent quantitative olfactory testing, which confirmed considerable hyposmia. Cranial magnetic resonance imaging was normal.
The patient was diagnosed with idiopathic PD. As he was only 55 years old and wanted to continue working, he chose pramipexole as first-line therapy, which has both anti-tremor and anti-depressive properties. Dopamine agonists are recommended as first-line therapy for patients such as this one, with early PD, moderate–severe motor disability, and lack of cognitive impairment (Figure 9).16


Unfortunately, after starting pramipexole (0.52 mg for 2 weeks and 1.05 mg thereafter) the patient’s tremor increased and he developed tension headaches. On examination he presented a left-sided dominant resting tremor and some loss of dexterity of his left hand. He felt depressed, was worried about his future and complained about rigidity-associated pain in his left arm when performing surgery. As electrocardiogram was normal, he was started on budipine 1 mg three times daily, which improved his resting tremor.
At the patient’s next appointment, he requested modification of his treatment as his colleagues had expressed concern about his ability to perform surgery. He also complained of dry mouth, which is a side effect of budipine. Budipine was therefore tapered and he was started on levodopa/carbidopa titrated to a final dose of 100 mg three times daily. Six months later the levodopa/carbidopa dose was increased to 150 mg three times daily, which allowed him to continue operating.
One year later the patient reported wearing-off and a Hauser home diary confirmed around 2 hours daily OFF time. In addition, PDQ-39 indicated impairments in the domains of mobility, activities of daily living, emotional wellbeing, stigma, communication and pain. He was started on safinamide 50 mg, taken in the morning to help with early-morning wearing-off. Within 4 weeks he showed marked improvements in motor skills (including dexterity and ambulation) and in his activities in daily living. Importantly, he reported less pain, less stigma and greater confidence about the future, both personally and professionally.
The patient remains on the 50 mg dose but this can be increased to 100 mg should he experience re-emergence of motor fluctuations in the future.
Summary and Conclusions
Safinamide is a unique therapeutic option in PD, with multiple mechanisms of action including MAO-B and dopamine reuptake inhibition, activity-dependent sodium-channel inhibition, and inhibition of abnormal glutamate release.2
Targeting both dopaminergic and non-dopaminergic mechanisms in PD is rational for two important reasons. First, most patients with PD eventually require levodopa in order to control their motor symptoms. Over time, and at high doses, levodopa inevitably results in motor complications, namely, fluctuations in treatment response and dyskinesias. At this point, adjunctive non-dopaminergic therapies are needed to help achieve control of motor symptoms and improve ON time, ideally without worsening dyskinesias. It is hypothesised that the effect of safinamide on dyskinesia may be explained by inhibition of state- and use-dependent Na+ channels and stimulated glutamate release. Second, whereas loss of dopamine-producing neurons in the substantia nigra is the primary pathologic defect in PD, non-dopaminergic pathways are also intimately involved in the pathogenesis of the disease. In particular, non-dopaminergic mechanisms underlie many of the non-motor symptoms of PD. These symptoms are frequently not recognised by the patients themselves or their physicians, despite being highly prevalent and having an equal, or greater, impact on quality of life as the cardinal motor symptoms.
As the cases described in this paper reveal, safinamide may be the best choice of add-on therapy in a wide range of patients with PD who are already taking levodopa. Safinamide has been evaluated in a comprehensive clinical study programme involving more than 3,000 patients, of whom more than 500 were treated for 2 years.1
In clinical studies involving patients with mid- to late-stage PD, safinamide demonstrated efficacy in improving ON time with no/non-troublesome dyskinesias in patients already taking levodopa; this effect was sustained to 24 months.3,4,12 Safinamide improved OFF time, motor complications, non-motor symptoms such as pain and mood, activities of daily living and quality of life.5,6 Importantly, safinamide is well tolerated with an adverse event rate similar to that of placebo.17
In conclusion, data from clinical trials together with real-world experience suggest that safinamide is the best add-on therapy in PD patients who require better control of motor and/or non-motor symptoms.














