**The expert faculty involved in this supplement were interviewed after the Bial-sponsored satellite symposium held at the 5th Congress of the European Academy of Neurology, Oslo, Norway, in June 2019. Their interviews, relating to the highlights of the symposium, have been included within the HTML version of supplement below, and were filmed with support from Bial.**
Introduction
Werner Poewe
Department of Neurology, Innsbruck Medical University, Austria
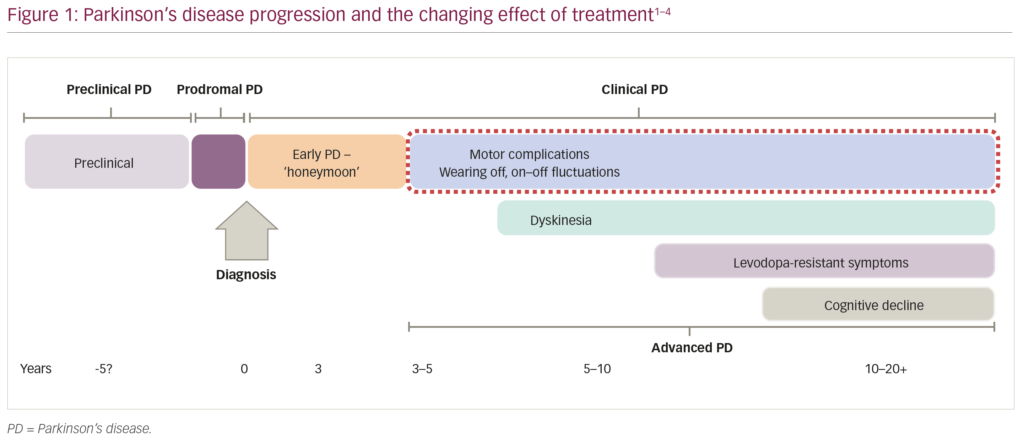
In the evolution of our understanding of Parkinson’s disease (PD), the current timeframe for the progression of the disease involves preclinical and prodromal stages, followed by an initial rewarding period of treatment following diagnosis (sometimes referred to as the ‘honeymoon period’), and over a few years the development of complications due to treatment responses wearing-off and levodopa-induced involuntary movements or dyskinesia (Figure 1).1–4 Here we will review the clinical spectrum of wearing-off symptoms, including both motor features and non-motor features, together with the pharmacological non-invasive treatment options for wearing-off. _EPUB-web-resources/image/1.png)
The spectrum of wearing-off – motor features
Angelo Antonini
Parkinson and Movement Disorders Unit, Neurology Clinic, University Hospital of Padua, Italy
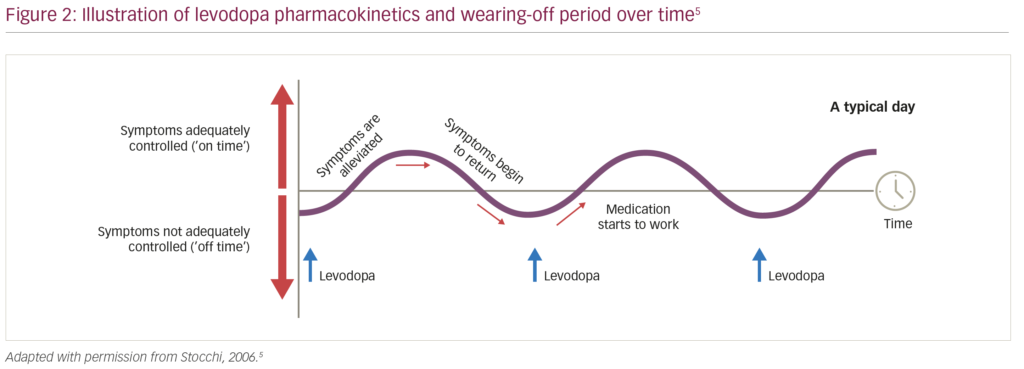
Wearing-off refers to the recurrence of motor (and non-motor) symptoms preceding scheduled doses of anti-parkinsonian medication, which typically improve immediately post-dosing (Figure 2).5 During wearing-off periods, motor symptoms are usually characteristic of PD (e.g., recurrent tremor, walking/balance impairment, slowness of movement), and tend to increase in severity as the disease progresses.1,5 Wearing-off is thought to be a result of the progressive degeneration of dopaminergic nerve terminals inherent in PD resulting in less uptake of exogenously administered levodopa and a shorter therapeutic response following dosing.6 However, increasing the dosing of levodopa to counter wearing-off also increases the risk of levodopa-induced dyskinesias; therein lies one of the key challenges in the management of PD. Wearing-off is also a common occurrence, with a study by the Parkinson’s Progression Markers Initiative showing that 4 years after their first PD therapy, 80% of patients experienced motor complications irrespective of the initial treatment (e.g., a dopamine agonist).7
Motor symptoms that occur during wearing-off periods can also exert a considerable burden on the patient’s functionality, activities of daily living and independence; data from the observational DEtection of wEaring off in Parkinson’s disease (DEEP) study (n=634) showed that patients with PD who experience wearing-off have significantly worse health-related quality of life (HRQoL; as assessed using the eight-item Parkinson’s Disease Questionnaire [PDQ-8])8 compared with patients who have stable PD (mean [± standard deviation] PDQ-8 score: 32.7 ± 19.2 versus
21.4 ± 15.7; p<0.0001).9 From the patient’s perspective, a UK study of 265 outpatients with PD showed that 28% of patients experiencing wearing-off rate a fluctuating response to medication as the most troublesome PD symptom.10 In addition, an online survey of 305 European patients with PD revealed that wearing-off affected almost all key daily activities, with the most commonly-affected and most bothersome (during wearing-off) being dressing, washing/keeping clean, effective communication, getting around the house and walking short distances.11 Reflecting this, the two strongest attributes driving patient preference for specific treatments were the duration of on-time (1 hour/day increase; odds ratio [OR] for preference: 1.40) and the predictability of wearing-off time (to within 30 minutes; OR: 1.42).11 Considering both the prevalence of wearing-off and the substantial burden exerted on the patient, it is important to detect wearing-off as early as possible so that appropriate therapeutic measures can be taken.
Several patient questionnaires have been developed to assess motor and non-motor symptoms and determine if a patient is experiencing periods of wearing-off. A task force, commissioned by the Movement Disorder Society, to assess the clinimetric properties of various questionnaires recommended the 19- and 9-item wearing-off questionnaires (WOQ-19 and WOQ-9) for diagnostic screening, and patient diaries (e.g., monitoring motor fluctuations and dyskinesia)12 for the assessment of wearing-off severity.13 Indeed, the WOQ-19 questionnaire has been shown to be more sensitive than neurologist assessments, particularly in the early stages of PD.9 Observational data from the DEEP study reported that wearing-off was diagnosed in 21.8% of patients by neurologists and 41.8% by the WOQ-19 after <2.5 years disease duration, 36.2% and 54.6% after 2.5–5.0 years disease duration, and 76.8% and 80.4% after >10 years disease duration, respectively.9 Nevertheless, there are still challenges in the clinical assessment of wearing-off that can lead to delays in detection and appropriate treatment. These include inter-patient variability in both the severity of functional impairment and the duration of wearing-off, as well as the presence of concomitant disabling features, such as pain, bladder dysfunction or mood changes that can alter a patient’s perception of wearing-off-related severity.13
In summary, wearing-off of motor control in patients with PD exerts a substantial functional burden on the patient, and efforts should be made to detect wearing-off as early as possible so that appropriate therapeutic action can be taken to mitigate symptoms. In addition to clinical assessment, the Movement Disorder Society recommends the use of the WOQ-19 and WOQ-9 questionnaires for detection of wearing-off, as well as patient home diaries to monitor motor fluctuations and dyskinesia.13 _EPUB-web-resources/image/1.png)
The spectrum of wearing-off – non-motor features
K Ray Chaudhuri
Parkinson Foundation International Centre of Excellence, King’s College Hospital, King’s College, London, UK
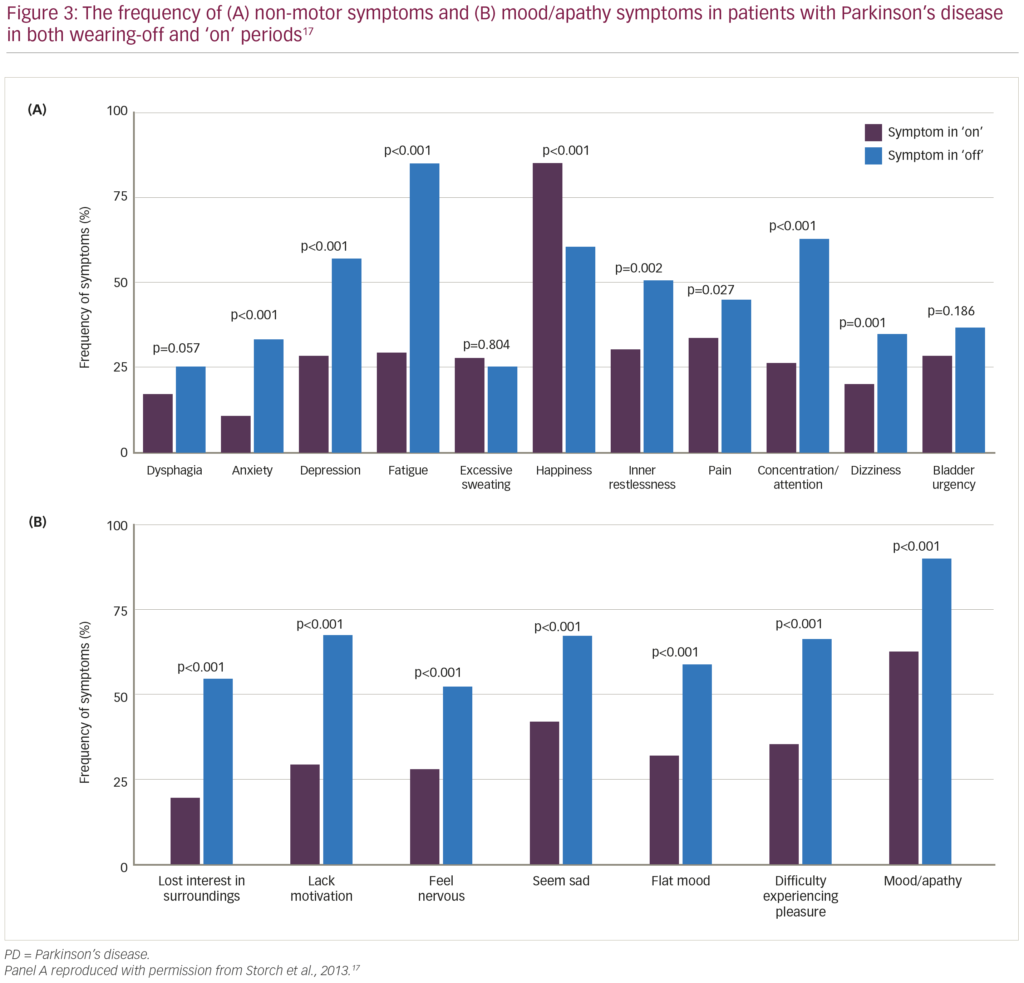
As well as the appearance of motor symptoms characteristic of PD, wearing-off has also been associated with a variety of non-motor PD symptoms.14 Historically, fluctuations in these neuropsychiatric and autonomic symptoms have been associated with fluctuations in motor symptoms.15 However, while most non-motor symptoms occur most frequently during wearing-off periods, there are some exceptions (e.g. euphoria, hyperactivity, hallucinations and some autonomic symptoms) that appear to be independent of wearing-off or occur more frequently outside of wearing-off periods.16 In a multicentre, cross-sectional study of 10 common non-motor fluctuations (dysphagia, anxiety, depression, fatigue, excessive sweating, inner restlessness, pain,
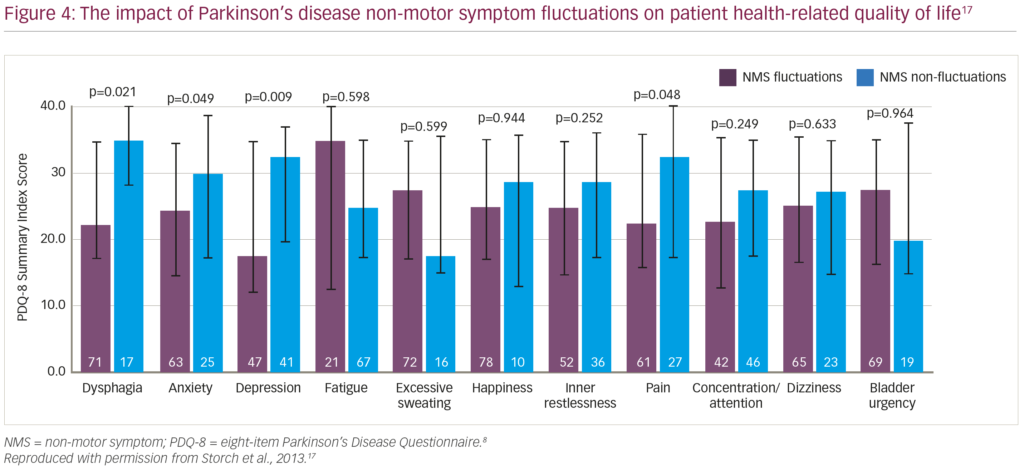
concentration/attention, dizziness and bladder urgency) in 100 patients with PD,17 all non-motor symptoms except dysphagia, excessive sweating and bladder urgency fluctuated in conjunction with motor symptoms (33–100% concurrence), with more frequent and severe symptoms occurring during wearing-off periods (compared with ‘on’ periods; Figure 3).17 The presence of anxiety, depression, fatigue and pain all had a negative impact on patients’ HRQoL (as assessed by the PDQ-8) independent of motor state, with fluctuations in anxiety, depression and pain significantly deteriorating HRQoL (Figure 4).17 Also, a European, multicentre, observational study revealed that early-morning wearing-off periods occur in 59.7% of patients with PD across all disease stages (44.3% in patients with Hoehn and Yahr stages 1.0–2.0; 68.9% with stages 2.5–3.0; and 63.5% stages 4.0–5.0), and that 88.0% of these periods were associated with adverse non-motor symptoms, predominantly urinary urgency (61.3%), anxiety (49.7%), dribbling (46.6%), pain (46.6%), low mood (45.5%), and limb paraesthesia (43.5%).18 Several of these non-motor symptoms were exclusive to the morning (pain [23.4%], urinary urgency [23.3%], anxiety [20.6%]), indicating that in many patients, early-morning wearing-off may be the most troublesome period and that prolonged or continuous drug delivery may help to alleviate this.18 This strategy was supported by research from Politis et al., who conducted a study where striatal dopamine levels in six patients with advanced PD were maintained over 24 hours using intestinal infusions of levodopa/carbidopa, resulting in improvement in both motor and non-motor symptoms.19
In summary, non-motor fluctuations during wearing-off periods are almost inevitable in patients also experiencing motor fluctuations, and vary from sensory and autonomic symptoms to disabling neuropsychiatric symptoms.17 In particular, patients rate response fluctuation to medication as the most bothersome problem in their lives,10 and early-morning, wearing-off-related non-motor symptoms can be very disabling.18 Treatments that prolong levodopa bioavailability and mimic continuous drug delivery may, therefore, be an effective strategy in the prevention and management of non-motor fluctuations.19 _EPUB-web-resources/image/1.png)
Non-invasive treatment options for wearing-off in Parkinson’s disease
María C Rodríguez-Oroz
Clinica Universidad de Navarra and Centre for Applied Medical Research, Pamplona, Spain
Recently, the International Parkinson and Movement Disorder Society Evidence-Based Medicine Committee reviewed their treatment recommendations for PD (last updated in 2011), with consideration given to new data from 143 Level I evidence studies investigating the treatment and management of motor symptoms, and 37 Level I evidence studies investigating non-motor symptoms.20 The Committee concluded that several adjunct therapies to levodopa were efficacious and clinically useful for the treatment of motor fluctuations in PD, including monoamine oxidase-B (MAO-B) inhibitors such as rasagiline and safinamide; catechol-O-methyltransferase (COMT) inhibitors such as entacapone, tolcapone and opicapone; and non-ergot dopamine agonists such as ropinirole, pramipexole, apomorphine and rotigotine.20
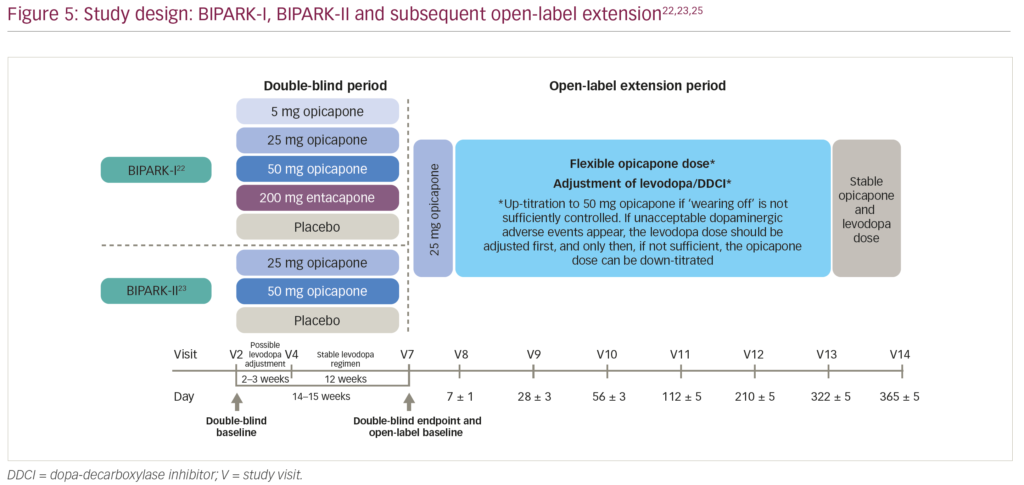
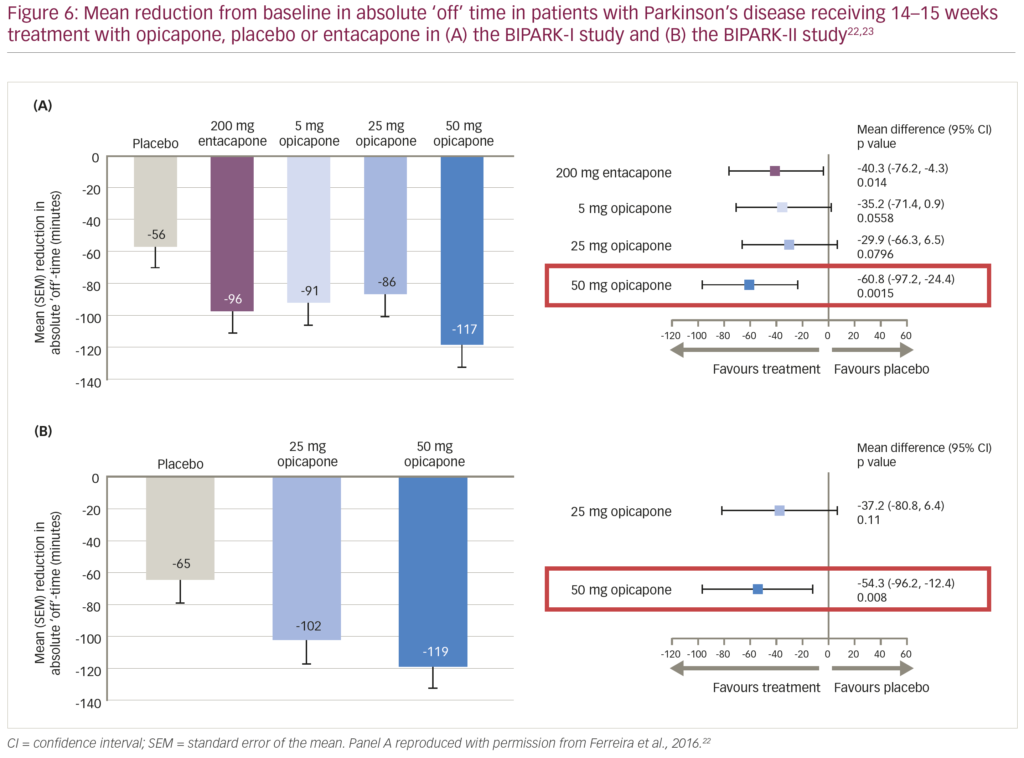
Opicapone is a long-acting, purely peripheral COMT inhibitor that has been shown to provide more sustained COMT inhibition and significantly greater levodopa bioavailability compared with entacapone with no associated liver toxicity.21 The clinical efficacy of opicapone as adjunct therapy to levodopa has been demonstrated in two phase III, multicentre, randomised, double-blind, active- and/or placebo-controlled clinical trials: BIPARK-I (n=600) and BIPARK-II (n=427).22,23 Patients with a clinical diagnosis of PD who had shown at least 1-year of clinical improvement with levodopa before at least 4 weeks of end-of-dose deterioration were randomised to receive 14–15 weeks of various doses of opicapone (5–50 mg), placebo or entacapone 200 mg (BIPARK-I only; Figure 5).22,23 The primary outcome for both studies was the change from baseline to the end of study treatment in absolute time in the off state.22,23 The change from baseline in absolute off time was significantly reduced in the opicapone 50 mg treatment group compared with placebo in both the BIPARK-I and BIPARK-II studies (difference: -60.8 minutes [95% confidence interval {CI} -97.2, -24.4; p=0.002] and -54.3 minutes [95% CI -96.2, -12.4; p=0.008], respectively; Figure 6)22,23 and non-inferior to entacapone in the BIPARK-I study (difference: -26.2 minutes [95% CI -63.8, 11.4; p=0.005]).22 In addition, opicapone 50 mg was well tolerated in both studies, with a similar rate of adverse event (AE)-related discontinuations compared to placebo (8.7% and 7.0%, respectively).24 Across both studies, the most commonly-reported AE in the opicapone treatment groups was dyskinesia, and only one death was reported (in the placebo group of the BIPARK-II study, due to pneumonia).24
Following the 14–15-week double-blind trials, patients from both BIPARK-I and BIPARK-II were then eligible to enter a 52-week open-label extension study (Figure 5).25 Upon entry into the open-label extension, patients were switched to opicapone 25 mg, up-titrated to opicapone 50 mg if wearing-off symptoms were not sufficiently controlled, and down-titrated only if unacceptable dopaminergic AEs occurred that could not be managed by levodopa dose adjustments.25 By the end of the 52-week extension, all patients showed a reduction of over 2 hours (120–130 minutes) in off time compared with double-blind study baseline values, irrespective of treatment during the double-blind phase.25 Patients treated with opicapone 50 mg during the double-blind phases maintained their efficacy during the open-label extension, whereas patients switching from placebo, entacapone, opicapone 5 mg and opicapone 25 mg showed further decreases in off time (-64.9, -39.3, -27.5 and -23.0 minutes, respectively).25 Overall, patients switching from entacapone who ended the open-label extension on opicapone 50 mg showed a significant reduction in off time of 68 minutes.25 Similar to the double-blind phases, the most commonly-reported AE during the open-label extension was dyskinesia (occurring in 14.5% of patients), which was effectively managed by adjustment of dopaminergic therapy.25 No new safety concerns were reported with long-term (>1 year) opicapone administration.25
In summary, PD symptom fluctuations as a result of wearing-off may be managed initially by levodopa fractionation, but as the disease progresses adjunct therapies to levodopa such as MAO-B inhibitors, COMT inhibitors and dopamine agonists can help to reduce wearing-off time when tailored to the individual needs of the patient.26 _EPUB-web-resources/image/1.png)
Summary
In PD, wearing-off is the term given to a predictable return of motor and non-motor symptoms that occurs before the next dose of anti-parkinsonian medication, and which typically resolves once the dose has been administered. As both motor and non-motor symptoms occur in most patients with PD during wearing-off,17 wearing-off exerts a substantial functional burden on the patient,1,9,17 and efforts should be made to detect wearing-off as early as possible so that appropriate therapeutic action can be taken to mitigate symptoms. Currently, the Movement Disorder Society recommends the use of the WOQ-19 and WOQ-9 questionnaires for detection of wearing-off, as well as patient home diaries to monitor motor fluctuations and dyskinesia.13 Treatments that prolong levodopa bioavailability and mimic continuous drug delivery may be an effective strategy in the prevention and management of motor fluctuations.19 Non-invasive measures to extend levodopa efficacy and reduce wearing-off time include adjunct therapies such as MAO-B inhibitors, dopamine agonists and COMT inhibitors such as opicapone, a long-acting, once-daily, peripheral COMT inhibitor.21
Overall, although our understanding of wearing-off has improved greatly over the years, challenges still remain and it is hoped that future research into assessment techniques, possible use of digital technology and next-generation therapies will further enable the early and effective treatment of wearing-off in patients with PD. _EPUB-web-resources/image/1.png)














