Phenomenology and clinical significance of OFF episodes in Parkinson’s disease
Werner Poewe
Department of Neurology, Innsbruck Medical University, Innsbruck, Austria
The emergence of OFF episodes in Parkinson’s disease (PD) marks the transition from the so-called ‘honeymoon’ period of good disease control to a phase in which motor fluctuations start to occur, approximately 3–5 years after the start of treatment. These fluctuations complicate treatment and are typically followed at later points by the emergence of dyskinesias (5–10 years), levodopa-resistant symptoms (10–15 years), and cognitive decline (15–20 years).1–4 Various retrospective, community-based, and young-onset PD studies indicate that the majority of patients with PD experience motor complications within 5–9 years of initial levodopa therapy.5–9 More recent randomized studies of levodopa and other treatments of PD, however, have found that motor complications can emerge much earlier; one such study showed that 17% of patients experience these symptoms after only 9 months of treatment (Table 1).10–12 Motor complications profoundly affect patients; in a study of 173 consecutive patients with advanced PD of more than 6 years duration at a treatment center in the UK, fluctuating response to medication was ranked the most troublesome symptom. This was above other PD-related symptoms including mood changes, drooling, sleep disturbance, tremor, pain, bowel problems, urinary problems, falls, and reduced appetite/weight loss.13 This study also found that among patients with either early or advanced PD, seven of the 10 most bothersome symptoms were of the non-motor variety.
The motor symptoms that are characteristic of OFF episodes in PD include rest tremor, rigidity, bradykinesia (which can appear as slowness, incoordination, reduced dexterity, weakness, and problems with gait), difficulty rising from a seated position, swallowing difficulty, balance problems, hypophonia, shortness of breath, or early morning muscle cramps (or dystonia) in hands, feet or legs.14,15 Wearing OFF describes the transition or deterioration from the ON state, when these symptoms are controlled by treatment, to the OFF state, usually at end of the dosing period when drug plasma levels have decreased to a critical level. Wearing OFF can occur rapidly or unpredictably, which increases the burden in PD and restricts the ability of many patients to participate in work or social activities.14
In PD, there are a range of non-motor symptoms that are specific to OFF periods in addition to the characteristic motor symptoms. Psychological symptoms include anxiety, panic attack, restlessness, irritability, mood changes, drowsiness, slow thinking, and depression. Other OFF symptoms include pain, tingling, sweating, being too hot or cold, urinary urgency, swallowing difficulties, and shortness of breath.16,17 In 2004, an international working group of leading movement disorder specialists reached a consensus definition of wearing OFF:
“A generally predictable recurrence of motor or non-motor symptoms that precedes a scheduled dose and usually improves with antiparkinsonian medication.”18
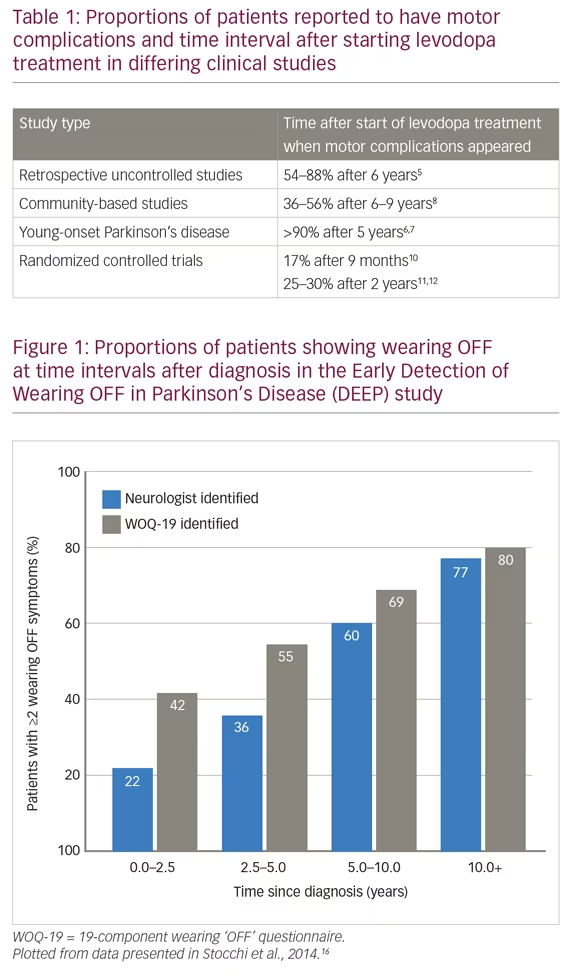
This definition recognizes the predictability of wearing OFF episodes and importantly, includes motor and non-motor symptoms, which form the clinical spectrum of PD. A 19-component wearing OFF questionnaire (WOQ-19) was developed by this working group and was used in the Early Detection of Wearing “OFF” in Parkinson’s disease (DEEP) study.16 This study included 617 patients diagnosed with wearing OFF (mean therapy duration was 6.6 years; 87% of patients were receiving levodopa) and found that wearing OFF was more frequently detected with the WOQ-19 than by neurological assessment (67% versus 57%). WOQ-19 was particularly sensitive for identifying these episodes in patients with PD of <2.5 years duration. This finding stresses the need for greater diligence in looking for wearing OFF even earlier in the disease course. In the DEEP study, wearing OFF was associated with younger age, female gender, higher Unified Parkinson’s Disease Rating Scale (UPDRS) part II score, and time since diagnosis (Figure 1). In addition, Parkinson’s Disease 8-item Questionnaire (PDQ-8) score, assessing quality of life, was correlated with the number of motor and non-motor wearing OFF symptoms.16 These findings support those of the Earlier versus Later Levodopa Therapy in Parkinson Disease (ELLDOPA) study (n=361), which found that the time to onset of motor complications and some dyskinesias was 5–6 months after starting levodopa treatment and that the proportion of patients with these symptoms was dose-dependent.10,19
For many years, the concept of wearing OFF and short duration of response to levodopa has been associated with rapid decreases in plasma levels within a few hours after each dose. This was demonstrated in an early pharmacokinetic study (n=16) that correlated plasma levels with disability in those receiving chronic levodopa therapy.20 The pharmacokinetics of levodopa are affected by many factors including swallowing problems, gastroparesis, Helicobacter pylori infection, competition with amino acids altering absorption, transport across membranes, and enzymatic conversion to dopamine.21 These factors may lead to delayed ON periods (due to absorption), end-of-dose wearing OFF episodes (short half-life of levodopa), unpredictable ON/OFF periods, partial ON periods, (possibly due to differing pharmacokinetics) or dose failure (no ON period).14,22
Delayed time to ON can comprise a substantial proportion of the total OFF time. This was emphasized in a study of 20 patients over 5 days, which found that mean time to ON was 46 ± 21 minutes, while the mean duration of wearing OFF was 21 ± 14 minutes.23 Additionally, there may be nocturnal recurrences of tremor, immobility, and akinesia during the extended interval after a patient’s final daily dose of levodopa. These may be particularly problematic on waking in the morning, a phenomenon known as early morning hypokinesia or akinesia, or early-morning OFF.9 An observational study of 320 consecutive patients with PD with varying disease severity (mild, 44.3%; moderate, 68.9%; severe, 63.5%) found that 59.7% had early-morning OFF episodes. Among the early-morning OFF episodes, 88% were also associated with non-motor symptoms.24 The most frequent non-motor symptoms during early-morning OFF episodes were anxiety, low mood, paresthesia, dribbling, urinary urgency, and notably, pain (possibly arising from involuntary movement).
Drug-induced dyskinesias can occur at various times during a dose cycle. Dyskinesia can occur during ON periods and involve phasic or dystonic movements. Biphasic dyskinesias can occur at onset or during wearing OFF and can have a mixture of phasic or dystonic movements. A characteristic form of dyskinesia is OFF period dystonia, which predominantly involves the feet or lower leg and can be intensely painful, requiring prompt treatment.22 Although these states are unpleasant, in a survey of 259 individuals with PD, patients who experienced dyskinesias (n=105) were significantly more likely to prefer dyskinesia over parkinsonian symptoms (i.e., the motor OFF state) than patients without dyskinesia.25
OFF episodes can be particularly distressing because they include both motor and non-motor symptoms. A cross-sectional study of 100 patients with advanced PD found that non-motor symptoms were heterogeneous and complex.17 It also found that anxiety, depression, fatigue, inner restlessness, pain, concentration/attention, and dizziness (determined by clinical examination) were all significantly more frequent (p=0.027–<0.001) during OFF compared with ON periods. All these symptoms, plus dysphagia and bladder urgency, were also more severe during OFF than ON periods (p=0.049–<0.001).
Motor symptoms in OFF episodes significantly impact quality of life. A study of 143 patients with PD found that OFF episodes significantly worsened the 39-item Parkinson’s disease quality of life questionnaire (PDQ-39) summary index.26 Nocturnal akinesia, specifically, worsened all PDQ-39 dimensions, including mobility, activities of daily living, emotional wellbeing, stigma, cognition, social support, communication, and bodily discomfort.
Summary
- Motor complications remain one of the most important limitations of long-term levodopa use.
- Underlying mechanisms of motor complications are mainly related to levodopa pharmacokinetics and absorption.
- Fluctuating response to medications, such as OFF episodes, ranks as the most troublesome symptom by patients.
- Clinical spectrum of OFF episodes includes motor and non-motor symptoms.
- OFF episodes negatively impact quality of life.
_Sunovion_EPUB-web-resources/image/1.png)
Pathophysiology and risk factors for the development of OFF episodes in Parkinson’s disease
C Warren Olanow
Department of Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Levodopa has been used as a treatment for PD for 50 years and remains the most effective therapy available. There are, however, limitations with levodopa, including a lack of control of non-dopaminergic features of PD such as falls and dementia, failure to stop disease progression, and the development of motor complications in the majority of patients.27 Risk factors for the development of these complications have been studied in both open-label and long-term prospective studies which indicate that both OFF time and dyskinesia are associated with young age, high doses of levodopa, and disease severity.8,28 Among these, levodopa dose is the one factor that can be controlled by physicians.
Interestingly, levodopa dose was also a major risk factor for the development of OFF periods. In the STRIDE PD study, for patients receiving <400 mg levodopa/day, the proportion experiencing wearing OFF was 27.2% compared with 72.6% for patients receiving >600 mg/day levodopa.28 These data indicate that if more levodopa is given, more OFF periods occur, suggesting there is a pharmacodynamic effect in addition to the pharmacokinetic effect influencing OFF periods. It could be argued that patients receiving higher doses of levodopa have more severe disease and are thus more likely to develop motor complications than those receiving lower doses. However, analyses using the C-statistic to evaluate the discriminative properties of a set of predictive factors determined that levodopa was a significant risk factor for both OFF time and dyskinesia when disease severity was excluded from consideration.28 This indicates that, independent of UPDRS score, higher levodopa dose significantly increases the risk of motor complications.
Analyses in this study further indicate that female gender and lower weight correlate with the development of motor complications; this likely reflects the same dose resulting in higher plasma levels in these individuals.28 Recommendations arising from this work suggest that physicians should use the lowest levodopa dose that provides satisfactory symptom control, should consider alternative medications to minimize levodopa dose, and should pay particular attention to the dose given to young women. It may also be necessary to consider patient weight and prescribe the dose on a mg/kg basis.
Increasing evidence now indicates that motor complications develop as a consequence of the non-physiologic restoration of brain dopamine with intermittent doses of standard oral levodopa/carbidopa.29 Studies show that levels of dopamine are normally maintained at relatively constant levels at all times and serve to stabilize the basal ganglia network and the selection of the desired movement whilst rejecting undesired movements. The stability of dopamine in the striatum is maintained in healthy rodent brains even following administration of a dose of levodopa. However, in the dopamine-lesioned rodent, basal concentrations of dopamine are pathologically low, and following a dose of levodopa, levels of dopamine in the striatum rise markedly.30 Thus, in the PD state, oral doses of levodopa are associated with abnormally high and low levels of dopamine.
This pulsatile stimulation of brain dopamine receptors leads to molecular changes in striatal input neurons, neurophysiologic changes in pallidal output neurons, and the development of motor complications.29 Based on these observations, it has been hypothesized that more continuous delivery of levodopa could restore brain dopamine in a more physiologic manner, avoid these effects and lessen the risk of developing motor complications. Animal models and clinical studies strongly support this hypothesis.
Primate studies showed that intermittent dosing of apomorphine (a dopamine agonist) via injection produced spikes in plasma levels and development of severe dyskinesia after only 7–10 days.31 Continuous apomorphine delivery of exactly the same dose via a subcutaneously implanted rod, however, produced stable plasma levels and no development of dyskinesia for periods up to 6 months.
Most importantly, evidence for the benefit of continuous levodopa treatment delivery came from a randomized study of patients with advanced PD (n=71).32 The study found that continuous intrajejunal infusion of a levodopa/carbidopa intestinal gel (Duodopa® or Duopa®, AbbVie Inc., North Chicago, IL, USA) for 12 weeks, significantly reduced OFF time and increased ON time without causing dyskinesia when compared with optimized treatment with intermittent doses of standard oral immediate-release levodopa/carbidopa. This study led to the approval of this treatment by the US Food and Drug Administration (FDA). The disadvantage of intrajejunal infusion is that it requires a surgical operation to insert the jejunal tube (with potential serious complications such as peritonitis, pancreatitis, and infection) and requires continuous infusion via a pump system. Associated tubing can become blocked, break, or leak and need replacement. This has led to an intensive search for other methods of trying to provide continuous levodopa delivery that avoids the need for a surgical procedure or an infusion system. The development of such a therapy could allow for a treatment that provides all of the benefits of levodopa without motor complications.
Summary
- Motor complications are associated with non-physiologic intermittent levodopa delivery.
- Higher doses of levodopa are associated with increased risk of motor complications (both OFF time and dyskinesia):
- lower doses of levodopa should be used when possible;
- levodopa dose should be increased incrementally; and
- polypharmacy should be utilized instead of increasing levodopa dose.
- Continuous delivery of levodopa offers the potential to prevent the development of motor complications.
_Sunovion_EPUB-web-resources/image/1.png)
Current approaches to the treatment of OFF episodes in Parkinson’s disease
Olivier Rascol
Research Network Departments of Clinical Pharmacology and Neuroscience, Toulouse University Hospital, Toulouse, France
The poor efficacy of levodopa in treating the OFF phenomenon in PD is caused by several pharmacokinetic issues including poor bioavailability, being only absorbed through the jejunum. Levodopa tablets can remain in the stomach for several hours due to issues with gut emptying, delaying the drug reaching the brain. In addition, levodopa has a short plasma elimination half-life of 60–90 minutes.33,34 The pulsatility of levodopa plasma levels dysregulates the cerebral and synaptic mechanism, generating post-synaptic abnormal plasticity and abnormal motor function.35,36 Objectives in PD treatment development have therefore been to find faster-acting drugs, to improve the bioavailability of levodopa, and to stimulate dopamine receptors in a more continuous manner. Approaches include:37
- alternate formulations of levodopa (e.g. Sinemet® CR [Mylan, Canonsburg, PA, and Sun Pharmaceuticals, Princeton, NJ, USA], soluble formulations, IPX066 [extended release formulation]) with longer duration of action;
- inhibitors of dopa decarboxylation at the periphery (e.g. carbidopa) to increase availability of levodopa in the central nervous system;
- inhibiting catechol-O-methyltransferase (COMT, e.g. entacapone, opicapone or tolcapone) to increase availability of levodopa in the central nervous system;
- inhibiting monoamine oxidase B (MAO-B, e.g. selegiline, safinamide or rasagiline) to reduce dopamine elimination;
- increasing dopamine release (e.g. amantadine, which acts via non-dopamine glutamate mechanisms); and
- dopamine agonists (e.g. apomorphine, bromocriptine, pramipexole, ropinirole or piribedil) to mimic dopamine.
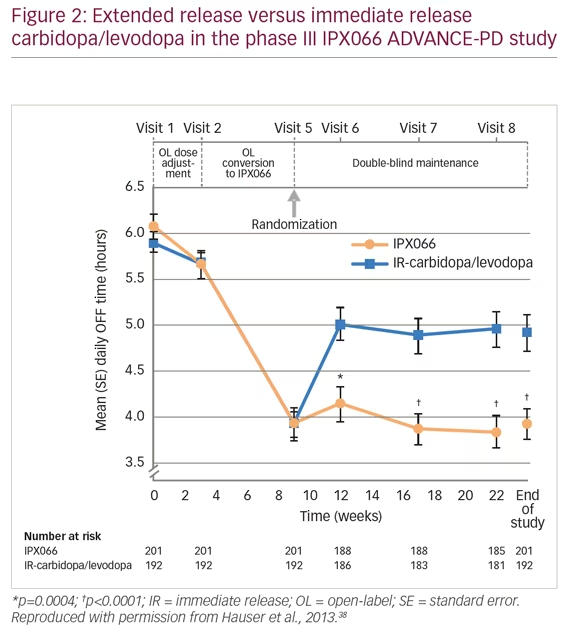
When patients with PD present with moderate wearing OFF episodes, it is first necessary to check that they are taking levodopa at the correct dose and time. It may be beneficial for patients to keep a diary to help identify when the OFF episodes occur and the possible causes. Physicians can adjust levodopa treatments, or add dopamine agonists, MAO-B inhibitors or COMT inhibitors. Adjustment in levodopa treatment was evaluated in the phase III, extended-release carbidopa/levodopa (IPX066) compared with immediate-release carbidopa/levodopa in patients with PD and motor fluctuations (ADVANCE-PD) study (n=471).38 Patients were treated with open-label, immediate-release carbidopa/levodopa for 3 weeks, followed by open-label extended-release carbidopa/levodopa formulation (IPX066) for a 6-week conversion period, and then randomized to further IPX066 or immediate-release formulation for 13 weeks. Patients receiving IPX066 in the randomized phase had a mean daily reduction of 1.17 hours in OFF time compared with immediate release (p<0.0001) (Figure 2). The most frequent adverse events were insomnia (3% versus 1%, respectively), nausea (3% versus 2%), and falls (3% versus 2%).
Dopamine agonists have also shown efficacy in reducing OFF time. The Clinical Efficacy of Pramipexole and Transdermal Rotigotine in Advanced PD (CLEOPATRA-PD) study (n=506) compared rotigotine 16 mg/day as a transdermal patch, pramipexole 4.5 mg/day orally, and placebo in levodopa-treated patients with advanced PD.39 The absolute change in OFF time from baseline versus placebo was -1.94 hours (p<0.0001) for pramipexole and -1.58 hours for rotigotine (p<0.0001). Responder rates, defined as patients with >30% reduction in absolute OFF time, were 67.0% for pramipexole, 59.7% for rotigotine, and 35.0% for placebo.
The MAO-B and COMT inhibitors have also shown efficacy in reducing OFF times. In the Lasting Effect in Adjunct Therapy with Rasagiline Given Once Daily (LARGO) study (n=687), patients were randomized to oral rasagiline (1 mg once daily), entacapone (200 mg with each levodopa dose) or placebo.40 Rasagiline and entacapone, respectively, changed mean daily OFF time by -1.18 hours and -1.2 hours versus placebo (p=0.0001, p<0.0001) and increased daily ON time (0.85 hours versus placebo 0.03 hours; p=0.0005 for both) without causing dyskinesia. Adverse event frequencies were similar with rasagiline (22%) and placebo (23%), but slightly higher with entacapone (27%).
A meta-analysis of 45 randomized trials involving nearly 9,000 patients found that adding different medications to levodopa treatment showed slightly differing reductions in OFF time:41
- dopamine agonists + levodopa changed daily OFF time by -1.57 hours versus placebo + levodopa (95% confidence interval [CI] -1.83, -1.31; p<0.001);
- COMT inhibitors + levodopa changed daily OFF time by -0.83 hours versus placebo + levodopa (95% CI -1.04, -0.62; p<0.001); and
- MAO-B inhibitors + levodopa changed OFF time by -0.93 hours versus placebo + levodopa (95% CI -1.25, -0.62; p<0.001).
The collected evidence indicates that early motor fluctuations can be managed by adding differing medications to levodopa but each has varying benefits and adverse event profiles (Table 2).
Alternative non-dopaminergic approaches to managing OFF episodes include A2A adenosine antagonists and N-methyl-D-aspartate (NMDA) antagonists. Istradefylline was recently approved by the US FDA for use as an adjunctive treatment to levodopa/carbidopa in patients with PD who experience OFF episodes.42 Studies of istradefylline show mixed results. A study by the Japanese Istradefylline Study Group (n=373) showed that istradefylline 40 mg/day significantly reduced OFF time compared with placebo (-0.96 hours versus -0.23 hours, p=0.003) but increased dyskinesia (12.1% versus 4.0%, respectively).43 Another study (EU-007, n=405), however, showed contrary results. After 16 weeks treatment there was a 0.70-hour reduction in OFF times for placebo compared with a 0.77-hour reduction for istradefylline (p=non-significant) versus a 1.22-hour reduction for entacapone (p<0.05).44 Amantadine, an NMDA glutamate receptor antagonist, has been shown to reduce OFF time. In a study of 75 patients, extended-release amantadine (274 mg ADS-5102, equivalent to 340 mg amantadine hydrochloride) used as an adjunct to levodopa, was shown to reduce OFF time by approximately 0.5 hours (treatment difference from placebo, -1.1 hours, p=0.0199), but also reduced dyskinesias.45
Overall, recent strategies to switch patients ON faster (reduce delayed ON), using oral drugs such as soluble levodopa, dopamine agonists, MAO-B and COMT inhibitors, have limitations. In addition, attempts to prolong dopaminergic antiparkinsonian responses using oral medications such as extended-release formulations, MAO-B, COMT inhibitors, and dopamine agonists have proven only partly effective. In the trials outlined above, baseline OFF periods are 5–6 hours/day with treatments providing reductions of 1–2 hours/day above a placebo effect of approximately 1 hour.38–41,43 This leaves 2–3 hours/day OFF time in many patients, with patients experiencing adverse events including dyskinesia, nausea, somnolence, impulse control disorder, hallucinations, and edema. This situation, especially in patients with severe fluctuations, prompts the use of second-line, more aggressive interventions. Subcutaneous apomorphine injections using a pen injector (e.g. Apokyn® pen, US WorldMeds, Louisville, KY, USA) are examples of this approach. In clinical trials, these injections have switched patients from OFF to ON within 20 minutes, with OFF abortion in 95% of patients versus 23% of patients for pen injector versus placebo (p<0.001), and significant reductions in UPDRS Part III score (p<0.001 and p=0.021 in different studies).46,47 Injections may be difficult to self-administer during an OFF period due to shaking and/or bradykinesia, and can be inconvenient when used in public situations.
In the Apokyn for Motor IMProvement of Morning AKinesia Trial (AM-IMPAKT, n=127) patients with delayed ON when receiving levodopa were switched to daily apomorphine injections (2–6 mg) for 7 days after titration to an optimal dose.48 This treatment significantly reduced time to ON from 60.86 ± 18.11 minutes to 23.72 ± 14.55 minutes (p<0.0001) and was well tolerated.
OFF periods in advanced PD can also be significantly reduced and ON periods increased by delivery of levodopa/carbidopa intestinal gel (Duodopa® or Duopa®) via a jejunal tube as discussed in the previous section. Strong clinical trial evidence supports this approach.32 However, the surgery necessary to insert the tube, associated infection risks, the need for continued medical supervision, and regular tube replacement make less invasive methods more desirable for many patients.
Another approach to reduce OFF time is continuous subcutaneous infusion of apomorphine using a pump. In the Apomorphine Subcutaneous Infusion in Patients With Parkinson’s Disease With Persistent Motor Fluctuations (TOLEDO) clinical study (n=107) over 12 weeks, continuous apomorphine infusion during waking hours significantly reduced OFF time (-2.47 hours) versus placebo (-0.58 hours; p=0.0025) and was well tolerated.50 The efficacy of this infusion approach emphasizes the need for continuous, rather than intermittent, drug delivery. In severe OFF episode treatment, however, the use of devices and pumps is limited. These therapies only reduce OFF time by approximately 2 hours/day, may have high costs, are complex to use and have tolerability issues. In addition, the optimal timing for their use and the most suitable choice between the currently available devices is not yet clear.
Alternative approaches to treating severe OFF periods include deep brain stimulation. In patients with advanced PD, a clinical study (n=156) has shown significant improvements after 6 months in quality of life (PDQ-39; p=0.02) and number of OFF periods (as measured by UPDRS-III scores, p<0.001) when comparing neurostimulation with medication versus medication alone.49 This approach, however, is restricted by costs and a greater incidence of serious adverse events including fatal intracerebral hemorrhage and the need for potentially hazardous surgery to position the electrodes.
Summary
- Different pharmacological approaches reduce OFF time in patients with PD and can be combined to increase efficacy.
- A proportion of patients still have several hours every day in the OFF condition despite treatments.
- Current device-based options are effective for reducing OFF time but have issues that limit their use.
- The management of OFF episodes is still a major unmet need for many patients with PD. Faster-acting and longer-lasting therapies are a necessity.
_Sunovion_EPUB-web-resources/image/1.png)
New therapies for the acute treatment of OFF episodes in Parkinson’s disease
Fabrizio Stocchi
Department of Neurology, IRCCS San Raffaele Pisana, Rome, Italy
The symptoms of OFF episodes can be caused by various factors including abnormal lingual control of swallowing and lingual festination. Patients with PD can also have a delayed swallowing reflex, which increases the risk of swallowing during inspiration, causing aspiration. Patients can also have a repetitive and involuntary reflux from the vallecula and piriform sinuses into the oral cavity.51 More importantly, many patients with PD have gastroparesis, which appears as postprandial bloating, early satiety, nausea, and vomiting.52,53 Delays in gastric emptying can cause slow delivery of levodopa to intestinal absorption sites, which, in turn, delays peaks in plasma levels leading to erratic drug responses, slow onset of action or dose failure.53–55 These issues were emphasized by gastroscopic examination of a patient, which found an intact levodopa/carbidopa tablet in the stomach 1.5 hours after it was swallowed.56 Furthermore, daytime gastroscopy has found food from the previous evening remaining in the stomachs of many patients with PD.
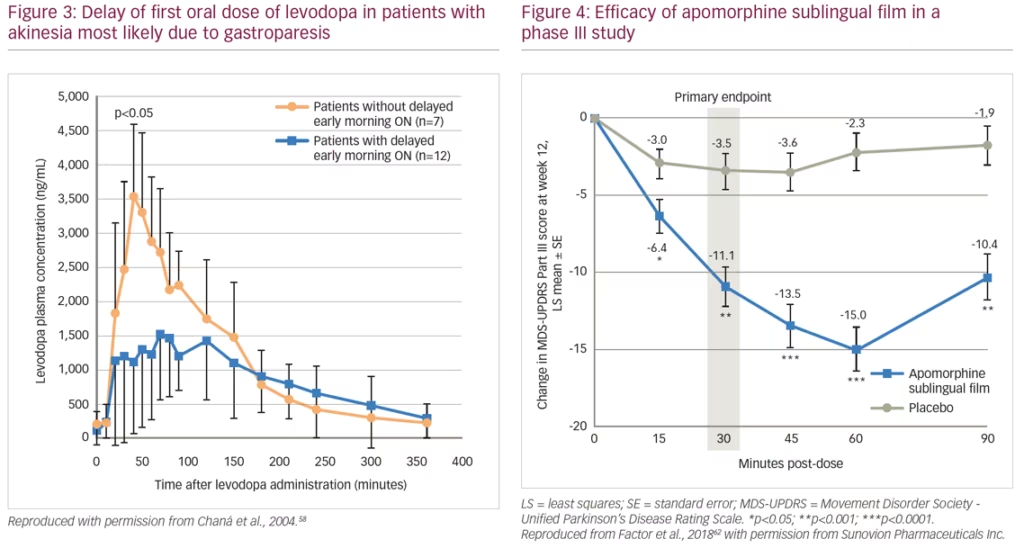
A pharmacokinetic study (n=6) showed that after oral doses of levodopa, patients show fluctuating plasma levels of the drug. After a dose of levodopa, there can be delayed peaks in plasma levels that are associated with delayed ON. Peaks in these levels are often rapidly followed by troughs, which are associated with severe wearing OFF.57 This study also showed that troughs in plasma levels can be reduced by continuous daytime intravenous infusion of levodopa rather than oral administration. In another study, patients (n=19) with morning akinesia (delayed ON) were found to have substantially lower peak plasma levodopa concentrations after levodopa administration than patients without morning akinesia (Figure 3).58 The difference in peak plasma levels was believed to be a result of delayed gastric emptying and not the levodopa absorption rate in the intestines.
Patients with delayed ON urgently need rescue therapy to allow for participation in daily activities. Rescue therapies augment maintenance therapies when required and are intended to provide rapid and predictable treatment for OFF episodes.59 Some rescue therapies, in addition to the pen injector device discussed above, are in various stages of development. Among these is an inhaled levodopa powder (CVT-301, Inbrija™, Acorda Therapeutics, Ardsley, NY, USA), which is administered in an inhaler device. It has recently been approved by the US FDA for use as a rescue medication in PD.60 The results of Safety and Efficacy of CVT-301 (Levodopa Inhalation Powder) on Motor Function During Off Periods in Patients With Parkinson’s Disease (SPAN-PD, n=351), a European phase III, randomized trial of the self-administered levodopa, CVT-301, have recently been reported.61 The findings show a significant benefit for CVT-301 over placebo for difference in UPDRS motor scores (the least-squares mean between-group difference in UPDRS motor score change during OFF periods for CVT-301 versus placebo was -3.92 [-6.84 to -1.00]; p=0.0088). The percentage of patients maintaining ON for 60 minutes at week 12 with CVT-301 84 mg was 58% versus 36% for placebo (p=0.0027). The treatment was well tolerated, it did not affect lung function, and there were few severe or serious adverse events. However, longer term studies of safety and efficacy of CVT-301 are needed.
An alternative investigational product for treatment of OFF episodes is an apomorphine sublingual formulation (APL-130277).62,63 This product consists of a film strip containing the drug that is placed under the tongue (without swallowing). In a phase I study of healthy volunteers, plasma levels of APL-130277 reached therapeutic levels similar to apomorphine subcutaneous injection.64 A phase III study (n=109) evaluated the use of APL-130277 in patients with idiopathic PD and OFF episodes. Least squares mean change in MDS-UPDRS Part III score from pre-dose to 30 minutes post-dose at 12 weeks was -11.1 for APL-130277 and -3.5 for placebo (p<0.0002) (Figure 4). Significantly more patients receiving APL-130277 achieved a self-rated full ON response within 30 minutes of a dose than placebo at week 12 (35% versus 16%, p=0.0426).62 APL-130277 was well tolerated and adverse events were generally mild. The most common adverse events with APL-130277 and placebo, respectively, were nausea (28% versus 4%), somnolence (13% versus 2%), and dizziness (9% versus 0%).63
Another investigational product for the treatment of OFF episodes is an intranasal device (INP103/POD®, Impel NeuroPharma, Seattle, WA, USA) that delivers levodopa to the highly vascularized upper nasal cavity. This method minimizes the amount of drug solution that drips from the nose or runs into the pharynx. Currently, a phase IIa study (n=24) is evaluating the use of INP103 in patients with idiopathic PD and OFF episodes who are stable on levodopa. Patients receive 35 mg, 70 mg, or 140 mg doses of INP103 or placebo. The primary endpoint is the safety and tolerability of single doses of INP103 in patients with PD during an OFF episode.65
These developments in rescue therapies have the potential to substantially improve quality of life and help patients deal with the otherwise untreatable symptoms of OFF episodes, which are a serious burden and involve both motor and non-motor symptoms.
Summary
- Many OFF episodes and delayed ON periods in PD are due to gastroparesis and other peripheral problems causing slow delivery of levodopa to intestinal absorption sites.
- Many patients with acute OFF episodes or delayed ON (especially in the morning) could benefit from rescue therapy that is delivered via a non-gastrointestinal route.
- New and investigational products delivering levodopa and other mediations via alternative routes include:
- an inhaled preparation/device of levodopa (CVT-301, InbrijaTM) that has shown efficacy and is well tolerated; it has recently been approved in the USA;
- an investigational sublingual film of apomorphine (APL-130277) that has completed phase III studies in the USA; and
- an investigational intranasal delivery system of levodopa (INP103/POD®) currently in a phase IIa study.
- New options for the treatment of OFF episodes have the potential to substantially reduce the burden of persistent OFF and delayed ON episodes, and restore patient confidence to participate in daily activities.
_Sunovion_EPUB-web-resources/image/1.png)
Conclusions
OFF episodes and delayed ON periods create a substantial burden in patients with PD and severely reduce their ability to lead normal lives.13,20–2 Current standard treatments, particularly the most effective maintenance medication—levodopa—when dosed on a routine basis, is frequently not able to prevent extended OFF times.21,66 Levodopa has been the mainstay of treatment for over 50 years and no alternative medications have proven to be more effective.37,67 Faster-acting and long-lasting medications that can reduce OFF time remain a substantial unmet need. The findings that intermittent levodopa treatment of PD tends to produce erratic plasma levels of the drug, causing alterations in the brain that produce motor fluctuations, have been key changes in the understanding of the disease and the effects of treatment in recent years.20,21 It has become clear that continuous maintenance of plasma levodopa levels to more closely resemble physiological conditions may be useful in preventing or reducing OFF periods.32 Efforts to develop systems for continuous intrajejunal delivery of levodopa,32 or subcutaneous infusion of apomorphine,50 are gathering pace, and their use has the potential to reduce recurrence of symptoms throughout the day.
The addition of drugs such as MAO-B or COMT inhibitors to levodopa to extend its half-life have been shown to reduce OFF times, but control of dyskinesias is still problematic. Deep brain stimulation may be another option to reduce OFF times, but its widespread use is limited by the need for invasive brain surgery and the associated risk of serious adverse events.49 Rescue medications for OFF episodes are another urgent unmet need in PD.59 The slow or failed action of oral drugs to alleviate symptoms is often the result of swallowing problems or gastroparesis delaying delivery of levodopa to intestinal absorption sites.52–55,68 To counter this, the development of products for non-gastrointestinal administration of medications is underway. Administration devices including pen injectors and inhalers have shown promising results, although the timing of their use and their relative merits need to be elucidated.46,47,61,63,69
In recent decades the understanding of PD and the pathophysiology of OFF episodes has advanced considerably.14,21,27,70 Whilst faster-acting and longer-lasting medications for treating symptoms are still needed, new formulations are under investigation. These developments have the potential to considerably diminish the burden and suffering that patients endure as a result of repeated OFF episodes. _Sunovion_EPUB-web-resources/image/1.png)