Parkinson’s disease is a progressive synucleinopathy that causes widespread neurodegeneration.1 It is estimated to affect approximately 6.1 million people globally and 0.7–1.0 million people in the USA.2–4 The incidence of the disease is expected to continue to increase over time, and with it, the burden to healthcare systems.3,4 The cardinal motor symptoms, which reflect nigral dopaminergic degeneration, are bradykinesia, rigidity, resting tremor, and later postural instability.5 Treatment with levodopa and other dopaminergic medications typically results in an initial robust improvement in motor symptoms.1,6 Degeneration in other brain areas leads to a multitude of non-motor symptoms, reflecting non-dopaminergic neurotransmitter derangements.7 Some non-motor symptoms are effectively treated by specifically addressing non-dopaminergic targets (e.g. serotonin, norepinephrine), while others are less understood and consequently poorly treated.7,8
While levodopa remains the cornerstone of Parkinson’s disease therapy,1,6,9 its use is limited by the emergence of motor complications, including periods of motor fluctuation, termed OFF episodes, and periods of dyskinesia.10–12 By 5 years, approximately 50% of patients have dyskinesia and/or OFF, and 80–90% have these symptoms by 10 years.13 Hence, as the neurodegenerative process progresses, the clinical response to levodopa becomes less predictable and more fragmented, with more frequent OFF episodes alternating with periods of ON with dyskinesia (non-troublesome and troublesome), both of which can adversely impact daily activities, health-related quality of life (HRQoL), care partner burden, and can result in additional healthcare costs.14–20
The underlying pathophysiology related to the emergence of motor complications is complex. OFF reflects variable gastrointestinal absorption of oral levodopa, short levodopa plasma half-life, striatal denervation with loss of buffering capacity, postsynaptic changes, and non-dopaminergic dysregulation of glutamatergic and adenosinergic pathways.12 Dyskinesia during ON occurs with suprathreshold plasma levodopa concentrations, serotonergic neuronal release of dopamine, and glutamatergic overactivity.21
Traditionally, the approach to managing OFF time has been based on increasing the dosage/frequency of levodopa and/or adding adjunctive dopaminergic medication, but these adjustments can cause an increased incidence of dyskinesia.22–26 In contrast, treatment of dyskinesia has centred on reducing levodopa and adding adjunctive dopaminergic therapy.27 The dopaminergic management of patients who experience both dyskinesia and OFF episodes can thus be problematic, creating a clinical conundrum of having to choose between reducing dyskinesia (with more OFF episodes) or improving OFF episodes (with increased dyskinesia).
Non-dopaminergic treatment with the low affinity, uncompetitive N-methyl-D-aspartate receptor antagonist amantadine was identified as a potential anti-dyskinetic therapy in the mid-1990s. Amantadine immediate-release (IR) (Symmetrel®; Novartis, Basel, Switzerland), indicated for parkinsonism and drug-induced extrapyramidal reactions, has shown some minor improvements in both ON time without dyskinesia and occassionally OFF time,28–35 suggesting that more appropriate and stable dosing of amantadine could provide clinical benefits. Two modified-release amantadine formulations have been assessed in clinical trial programmes designed to determine their anti-dyskinetic properties. Gocovri® (Adamas Pharmaceuticals, Emeryville, CA, USA) is a once-daily, night-time, modified-release formulation containing coated pellets that result in an initial delay in exposure, followed by extended release (ER) over the dosing interval (hereafter referred to as amantadine ER(Go)).36 Osmolex® ER (ER(Os)) (Adamas Pharmaceuticals, Emeryville, CA, USA) is a once-daily, morning-administered modified-release formulation constisting of an IR layer with an ER core.
Amantadine ER(Go) was specifically developed as a glutamatergic treatment for dyskinesia to address the dopaminergic dilemma between OFF and dyskinesia.37 Taken at bedtime, this particular ER formulation provides higher plasma levels of amantadine during the waking day, and lower levels overnight,37 with the intent of minimizing adverse effects on sleep.38,39 Based on the results of two large, randomized, placebo-controlled pivotal trials, amantadine ER(Go) received regulatory approval by the US Food and Drug Administration (FDA) in 2017 for levodopa-induced dyskinesias in Parkinson’s disease with or without concomitant dopaminergic therapy, and was granted an additional indication in 2021 as an adjunctive treatment to levodopa/carbidopa for the treatment of OFF episodes in Parkinson’s disease.36,40,41 In contrast, both amantadine IR and amantadine ER(Os) share an indication from the FDA for Parkinson’s, disease but not for OFF episodes or dyskinesia.39,42
This review will discuss the impact of dyskinesia and OFF, the clinical development of amantadine ER(Go), and will outline treatment strategies to increase ‘good’ or ‘functional’ ON time by minimizing both OFF time and time spent with troublesome dyskinesia.
The impact of dyskinesia and OFF in Parkinson’s disease
Dyskinesia and OFF can lead to functional impairment and disability, and, consequently, have a large impact on patient HRQoL and daily living.14–16 A survey of 683 patients with Parkinson’s disease found that the severity and duration of dyskinesia were significant predictors of poor HRQoL.43 The most strongly affected elements of HRQoL are reported to be mobility, stigma, communication, cognition and activities of daily living.14 Impact on activities of daily living is particularly common, with a pooled analysis of clinical studies involving patients with Parkinson’s disease and troublesome dyskinesia finding that approximately two-thirds of patients reported experiencing at least mild impact on six out of the ten activities of daily living included on the Unified Dyskinesia Rating Scale (UDysRS) part 1B.16 These included walking and balance, being in public and social settings, exciting or emotional settings, engaging in hobbies and other activities, handwriting, and dressing. Additionally, physicians found that dressing was the activity of daily living most commonly impacted, with 64% of patients having at least moderate impairment.16
The HRQoL impact of motor complications on walking extends beyond limitation to movement, as patients with dyskinesia and those in the OFF state have an increased risk of falls, leading to injury and an associated further reduction in HRQoL.44,45 There is also an association between the time patients spend OFF and increased care partner burden, including anxiety and having insufficient time for oneself, particularly when OFF episodes occur during the morning.17,18 Dyskinesia has also been linked with undernutrition and weight loss.46 Furthermore, the development of dyskinesia has been associated with a two-fold increase in Parkinson’s disease-related costs.19 In patients who spend more than 75% of their waking hours per day OFF, there was 2.4-fold increase in total costs compared with those spending fewer than 25% of their time OFF, mostly driven by increases in professional and informal care.20
Another impact of motor complications can arise from treatment strategies aimed at reducing the risk of their emergence. Due to the association between increasing levodopa dose and increasing incidence of motor complications, including dyskinesia and OFF, a historical levodopa therapy aversion still persists among some physicians and patients. Levodopa-sparing strategies have been employed early in the disease to attempt to reduce the potential for dyskinesia development.11,47,48 However, the LEAP (Levodopa in EArly Parkinson’s Disease) trial demonstrated no beneficial or detrimental disease-modifying effect of levodopa-sparing strategies in patients with early Parkinson’s disease over 80 weeks.49 Additionally, the use of levodopa-sparing strategies in early-stage Parkinson’s disease is associated with higher therapy costs, more frequent adverse events (AEs) and increased risk of worsening motor symptoms such as bradykinesia.27,50 Together, these issues highlight the need for treatment options to reduce the burden of dyskinesia and OFF in patients with Parkinson’s disease.47
Pharmacological options for treating dyskinesia and OFF episodes in Parkinson’s disease
The goal of treating OFF episodes is to maintain a functional or good ON state without troublesome dyskinesia, i.e. improving ON time while avoiding an increase in troublesome dyskinesia. Until the recent approval of amantadine ER(Go), there were no pharmacological treatments demonstrated to improve both dyskinesia and OFF. Consequently, treatment options to address these symptoms have attempted to balance time spent ON with troublesome dyskinesia and time spent in an OFF state.27
Treatment guidelines for motor fluctuations and dyskinesia from the Movement Disorder Society (MDS) and the American Academy of Neurology (AAN) have recommended adjusting levodopa dose and/or adding adjunctive therapies.6,51 Strategies involving the adjustment of levodopa dose include either levodopa fractionation or increasing levodopa dose, but many accept inadequate symptom control to avoid exacerbating dyskinesia.14 The use of levodopa ER and continuous administration formulations, which aim to minimize fluctuation in levodopa concentrations, have been demonstrated to decrease OFF time and are rated as ‘likely efficacious’ in reducing dyskinesia severity, although these treatments themselves still frequently cause dyskinesia as an AE.6,52–54
A recent post-hoc analysis of the GLORIA registry (Global Long-term registry On efficacy and safety of Rescue levodopa‐carbidopa Intestinal gel in patients with Advanced Parkinson’s disease in routine care) found that dyskinesia was only reduced in patients with more than 4 hours per day of dyskinesia and not eliminated by continuous intrajejunal levodopa in any group of patients.55 Adjunctive treatment strategies can improve OFF but may increase dyskinesia. These adjuncts include dopamine agonists, monoamine oxidase-B (MAO-B) inhibitors and catechol-O-methyl transferase (COMT) inhibitors,6,9,27 and were deemed ‘efficacious’ or ‘clinically useful’ for reducing OFF time by the latest update of the MDS evidence-based review in 2018,6 and adenosine 2A receptor antagonists may be ‘possibly useful’.6 However, as with alternative levodopa formulations, all these adjunctive treatments for OFF increase dyskinesia in 8–30% of patients, often necessitating levodopa dose reduction.22–26
Other treatment approaches for dyskinesia have been tried. The dopamine agonist pramipexole has been investigated, but evidence of efficacy is limited to a single study that examined the effect of patients switching their dopamine agonist to pramipexole versus those adding it to their existing therapeutic regimen.6,56 Additionally, two patients out of 18 in the study demonstrated a worsening of dyskinesia, a known potential effect of pramipexole reflected in the product label.56,57 Other investigated treatments including zonisamide and levetiracetam have not demonstrated sufficient evidence to be considered clinically useful.6
Amantadine in the treatment of dyskinesia and OFF in Parkinson’s disease
Amantadine IR formulation was recommended as efficacious and clinically useful for the treatment of dyskinesia by the MDS evidence-based review in 2018, and possibly effective by the AAN guidelines.6,51 This recommendation was based on several randomized, double-blind studies.28–35 Goetz et al. demonstrated reductions from baseline of 10 points in UDysRS after 8 weeks of amantadine IR versus placebo treatment in addition to improvements on several other dyskinesia scales, although not Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part IV, in 68 patients with dyskinesia.28 A crossover study (n=36) by Sawada et al. demonstrated that after approximately 4 weeks of treatment, 64% versus 16% of amantadine IR- and placebo-treated patients, respectively, experienced an improvement in dyskinesia measured using the Rush Dyskinesia Rating Scale, in addition to a 2-point reduction in UPDRS (now known as the MDS-UPDRS), part IVa (dyskinesia) scores.29 Changes in UPDRS-IVb scores for OFF motor fluctuations were not significantly different between treatments.29 Similarly, a levodopa challenge cross-over study (n=24) by Snow et al., found a 1.1-point reduction in UPDRS-IVa scores after approximately 3 weeks of amantadine IR versus placebo treatment.30 A longer-term follow-on study to a previous cross-over study by Metman et al., found that reductions in dyskinesia and the proportion of the waking day spent OFF (0.5-point reduction), measured by UPDRS IV item 39 scores following 3 weeks of amantadine IR, were maintained in 13 of 17 patients with motor fluctuations and dyskinesia still taking amantadine after 1 year.31,32
Finally, three studies have also examined the effect of amantadine IR on dyskinesia using a withdrawal methodology.33–35 Thomas et al. (n=40) found initial improvements from baseline in dyskinesia and OFF time (-1.6 hours) after 1 month of amantadine IR treatment, compared with no change in the placebo group.33 However, improvements were not sustained beyond more than 8 months of amantadine IR treatment and withdrawal was associated with rebound dyskinesia.33 Wolf et al. demonstrated that double-blind withdrawal of amantadine IR resulted in a statistically significant (p=0.02) increase from baseline in dyskinesias for patients with motor complications due to levodopa switched to placebo for 3 weeks (mean duration of previous amantadine IR treatment: 4.8 years).34 In comparison, there was no change in dyskinesia in patients randomized to continue amantadine IR; treatment differences in this effect were not statistically significant, potentially due to the small size of the included population (n=32).34 The more recent randomized, placebo-controlled, double-blind, washout AMANDYSK (AMANtadine for DYSKinesia) trial (n=57) found that following 6 months of amantadine IR treatment, amantadine withdrawal for 3 months was associated with a significant worsening in ON time with troublesome dyskinesia, and a numerically lower reduction in OFF time (treatment difference: 0.3 hours; p=0.70).35
Overall the evidence for efficacy of amantadine IR in dyskinesia has been limited by small sample sizes (n=18–68) and heterogeneity in study methodology, with several trials using uncontrolled or non-randomized designs, leading to inconsistent ability to demonstrate sustained efficacy. Only one trial by Metman et al., showed a significant effect of amantadine IR on OFF.28–35,58 Furthermore, tolerability of amantadine IR when given at higher doses (300–400 mg), which may provide greater anti-dyskinetic effects than lower doses (100–200 mg),32 is poorer and associated with a greater incidence of AEs.38
More recently, amantadine ER(Go) has offered the prospect of higher, therapeutic amantadine plasma concentrations on awakening that are sustained throughout the day.37 Amantadine ER(Go) is the only drug approved by the FDA to treat dyskinesia in the USA, and is the only treatment demonstrated in two large, randomized, placebo-controlled trials to be clinically effective at reducing both OFF time and dyskinesia.
The amantadine extended-release Gocovri clinical development programme
The amantadine ER(Go) development programme included non-clinical toxicology studies to meet current standards, and pharmacokinetic studies, in addition to the following clinical studies: the phase II/III EASED study (Extended release Amantadine Safety and Efficacy study in levodopa-induced Dyskinesia), which was a dose-finding study;59 the pivotal EASE LID and EASE LID 3 studies (Extended release Amantadine Safety and Efficacy studies in Levodopa-Induced Dyskinesia);40,41 and the 2-year, open-label EASE LID 2 study.60 An overview of these studies is included in Table 1. Further data have also been generated from pooled analyses of the phase III EASE LID and EASE LID 3 studies focusing on efficacy and safety,61,62 using a novel examination of diary events,63 and activities of daily living,16 in addition to a pharmacokinetic analysis of two phase I trials and the EASED study.37 Together, these studies have provided extensive data on amantadine ER(Go) in patients with dyskinesia and OFF.36
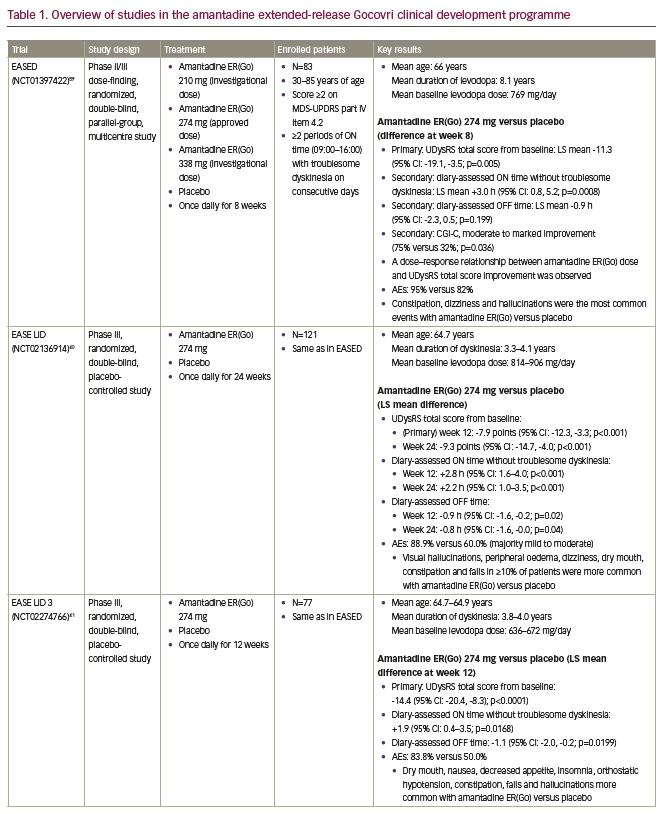
The amantadine ER(Go) development programme included approximately 280 patients treated with amantadine or placebo, providing a large population to assess amantadine’s safety profile in patients with Parkinson’s disease and dyskinesia with motor complications.40,41,59 Another feature of the amantadine ERGo, EASED, EASE LID and EASE LID 3 studies was the use of a pre-specified testing hierarchy for endpoints. In each study, treatment differences on the primary endpoint were assessed before further stepwise comparison of treatment differences for secondary endpoints, controlling for type one error. The primary endpoint in these studies was the UDysRS, a validated clinical rating scale that combines clinician-observed and patient-rated measures of dyskinesia impact on function.28,40,41 An analysis of available dyskinesia scales found that only the UDysRS was sensitive to changes in dyskinesia severity with treatment.28 Additionally, a lack of sex, age, race/ethnicity and educational influence on the scale has been shown.64 The minimal clinically important difference for this scale is a 10-point change from baseline.65 In addition to the UDysRS, the EASE LID studies also included endpoints based on patient diaries and the MDS-UPDRS, allowing for the assessment of changes in dyskinesia and OFF time by multiple methods.40,41
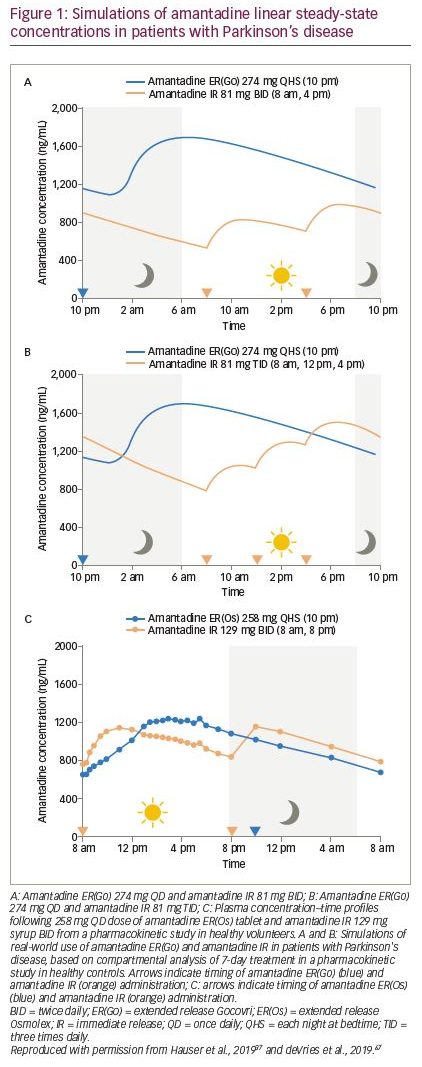
Amantadine ER(Go) is taken once daily at bedtime and results in an initial delay in absorption followed by a slow initial rise in concentration, so that concentrations are near maximum level around the time of wake-up (Figure 1A and B).37,66,67 Consequently, it provides peak drug concentrations from the morning and continuous coverage throughout the day, while minimizing night-time exposure.37,66,67 In contrast, conventional administration of amantadine IR two or three times daily results in lower plasma concentrations earlier in the day and higher concentrations at night (Figure 1A and B). The plasma concentration–time profile of amantadine ER(Os) and amantadine IR is shown in Figure 1C. Higher amantadine concentrations at night may be undesirable given the potential for amantadine to cause sleep disturbances.38,39 Pharmacokinetic modelling has demonstrated that amantadine ER(Go) and amantadine IR differ in terms of drug exposure at various times over a dosing interval, with the approved amantadine ER(Go) dose of 274 mg once daily, providing 1.4–2.0-fold higher daytime amantadine plasma concentrations versus amantadine IR 81 mg two or three times daily.37
Amantadine extended-release Gocovri in randomized, controlled clinical studies
The first study to investigate the efficacy and safety of amantadine ER(Go) in patients with Parkinson’s disease was the phase II/III randomized, double-blind, parallel-group, dose-finding EASED study (ClinicalTrials.gov Identifier: NCT01397422).59 Patients received investigational doses of amantadine ER(Go) 210 mg (n=20), 274 mg (n=21; approved dose) or 338 mg (n=20) (equivalent to amantadine hydrochloride salt 260, 340 and 420 mg, respectively) or placebo (n=22) once daily at bedtime for 8 weeks. Recruited patients were 30–85 years of age with Parkinson’s disease, were taking levedopa ≥3 times daily and experiencing dyskinesia with at least mild impact on activities (i.e. an MDS-UPDRS part IV item 4.2 score ≥2) and had at least two periods of ON time with troublesome dyskinesia per day between 09:00 and 16:00 on consecutive days. Troublesome dyskinesia was defined as dyskinesia that interferes with function or causes meaningful discomfort, as determined by the patient. Key exclusion criteria included atypical Parkinson’s disease, levodopa or dopamine agonist-induced psychosis, cognitive (Mini-Mental State Examination score <24) or renal impairment (estimated glomerular filtration rate [eGFR] <50 mL/min/1.73 m2), and a history of deep brain stimulation (DBS).
Key study results are shown in Table 1. In brief, 8 weeks of amantadine ER(Go) treatment significantly improved the primary endpoint of UDysRS total score in a dose-dependent manner compared with placebo (least squares [LS] mean difference: -11.3 points). Amantadine ER(Go) 274 mg increased diary-assessed ON time without troublesome dyskinesia, numerically reduced OFF time and resulted in more patients having moderate to marked improvement in Clinicians Global Impression of Change (CGI-C) versus placebo (Table 1). Other secondary endpoints, including fatigue, MDS-UPDRS (parts I, II, III) total score and Parkinson’s Disease Questionnaire, were not significantly different between treatment groups.59
The safety profile of amantadine ER(Go) was acceptable; AEs of constipation, dizziness and hallucinations were more common with amantadine ER(Go) versus placebo, and no effect on sleep duration was observed.59 Despite good efficacy, withdrawals due to AEs were most common at the highest 338 mg amantadine ER(Go) dose, suggesting that the 274 mg dose provided the best benefit–risk profile.
Both the EASE LID (ClinicalTrials.gov Identifier: NCT02136914) and EASE LID 3 (ClinicalTrials.gov Identifier: NCT02274766) phase III, randomized, double-blind, placebo-controlled studies investigated the efficacy of amantadine ER(Go) 274 mg once daily versus placebo.40,41 EASE LID and EASE LID 3 included 121 and 77 patients treated for up to 24 and 12 weeks, respectively, with the same eligibility requirements as the EASED study. Consistent with the EASED study, both studies demonstrated significant reductions in the impact and amount of daily time spent with dyskinesia assessed by the UDysRS (LS mean difference: -9.3 points [week 24] and -14.4 points [week 12], respectively) and patient diaries with amantadine ER(Go) 274 mg treatment throughout the study and at the final clinical visit. Moreover, OFF time was significantly reduced in both the phase III studies (Table 1). The anti-dyskinetic effect of amantadine ER(Go) was also supported by significant improvements in the MDS-UPDRS part IV score, assessing motor complications. Amantadine ER(Go) did not worsen motor function, as suggested by MDS-UPDRS part III scores. Both studies demonstrated that more patients treated with amantadine ER(Go) had marked improvements in physician-assessed patient functioning, as measured by CGI-C score. Safety results for amantadine ER(Go) were generally consistent with those of the EASED study, although the incidence of visual hallucination with amantadine ER(Go) was lower in EASE LID 3 than in EASE LID (8% versus 24%).40 This may have been due, at least in part, to the lower mean levodopa dose at baseline, and shorter study duration in EASE LID 3 versus EASE LID.40,41
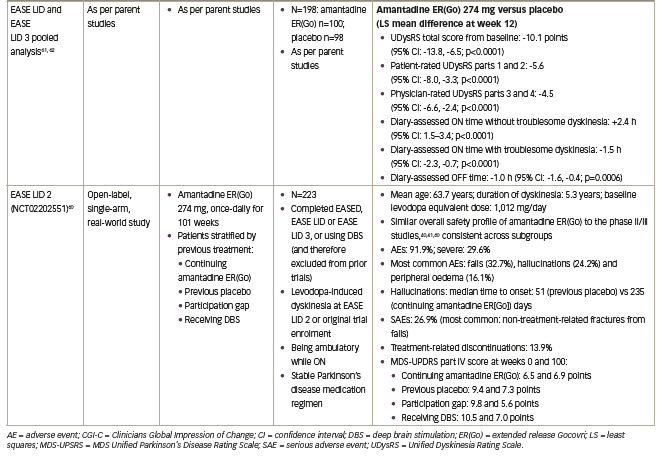
Further pooled and post-hoc analyses of pivotal amantadine extended-release Gocovri clinical studies
The benefit of amantadine ER(Go) in reducing dyskinesia and OFF time in Parkinson’s disease was assessed in a pooled analysis of the EASE LID and EASE LID 3 studies, which both had very similar study designs, and were conducted using the same operating and training procedures by the same research organizations.16,61–63 This analysis provided a larger population in which to examine the safety of amantadine ER(Go), more precise estimates of efficacy, and the assessment of result consistency between patient subgroups. In addition, it allowed for further analyses of exploratory endpoints such as the effect of amantadine ER(Go) on patient diary-recorded OFF time and dyskinesia. However, it should be noted that these analyses for certain exploratory endpoints, such as item analyses of rating scales and patient populations, such as safety in elderly patients, are considered hypothesis generating and were not specifically powered to evaluate treatment differences.
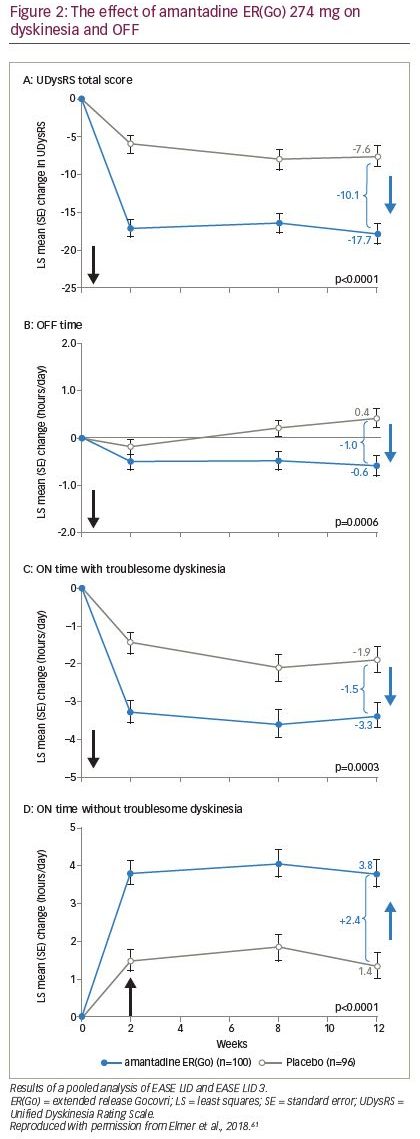
The first pooled analysis included 100 patients who received amantadine ERGo and 96 patients who received placebo, and confirmed that improvements in troublesome dyskinesia, measured by UDysRS, were seen by the first on-treatment efficacy clinical visit (week 2) and sustained through week 12; improvements in OFF time were observed from week 8 and sustained through week 12 (Figure 2; Table 1).61,62 Improvements in UDysRS (difference: -10.1 points) at week 12 with amantadine ER(Go) (-17.7) over placebo (-7.6) exceeded the 10-point minimum clinically important difference for the UDysRS, calculated based on amantadine ER(Go) trial data;65 improvements were consistent across both patient- and physician-rated parts of the UDysRS and across several subgroups of patients based on sex, age and dyskinesia severity. In addition, improvements in Parkinson’s disease diary outcomes with amantadine ER(Go) versus placebo included a 1 hour/day decrease in time spent OFF, a 1.5 hour/day decrease in ON time with troublesome dyskinesia and a 2.4 hour/day increase in ON time without troublesome dyskinesia (Figure 2; Table 1). Improvements in MDS-UPDRS part IV total score and items 4.1–4.4, which assess impact and time spent with dyskinesia and OFF, were also confirmed. Additionally, twice as many patients receiving amantadine ER(Go) versus placebo (76.0% versus 37.5%) experienced an improvement in symptoms as rated by CGI-C score. There were no new safety findings, although the analysis of pooled data demonstrated that hallucinations were more common in patients ≥65 versus <65 years of age and in those with an eGFR of 50–89 versus ≥90 mL/min/1.73 m2.61
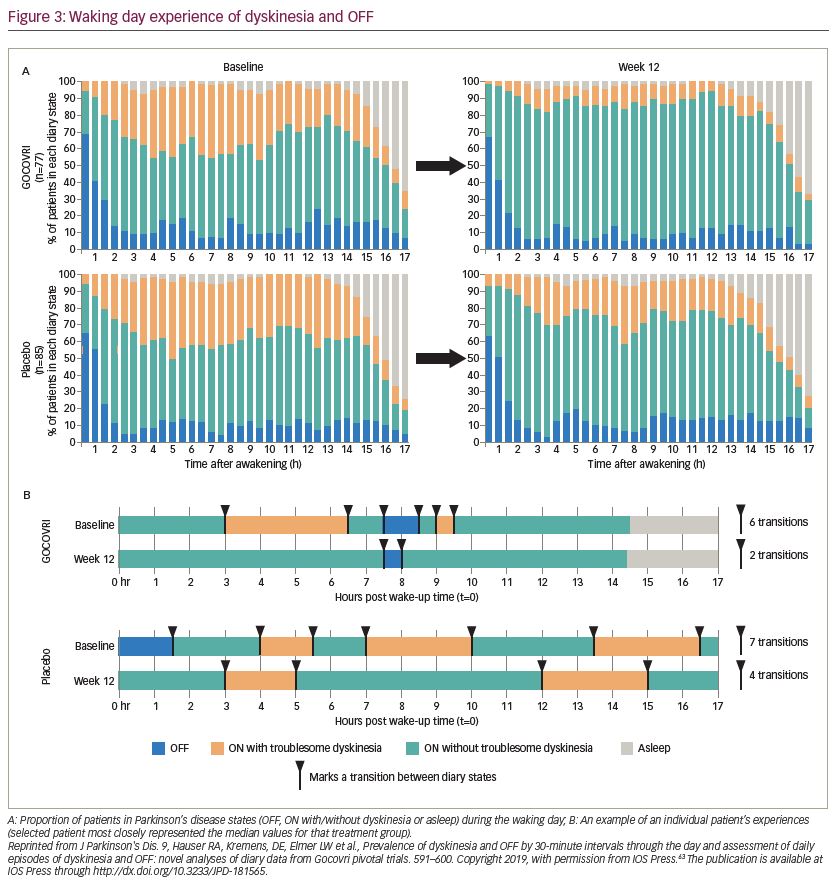
The effect of amantadine ER(Go) on dyskinesia and OFF time was explored in another pooled analysis of individual Parkinson’s disease diaries from 162 EASE LID and EASE LID 3 patients with diary entries from baseline and week 12.64 In both studies, patients categorized their predominant motor state during 30-minute intervals throughout the day. In this analysis, the total number of transitions between motor states and duration of each motor state episode were determined at baseline and with treatment. Before treatment, 67% of the patient sample woke up OFF and approximately 12% of the patient sample were in an OFF state during any subsequent 30 minute period after the first 2 hours following waking up (Figure 3A). Amantadine ER(Go)-treated patients had an additional 3 hours per day of continuous ON time without troublesome dyskinesia and 2.2 fewer transitions between motor states than placebo-treated patients (baseline: 8.1 transitions per day) (Figure 3B). Overall, patients receiving amantadine ER(Go) had decreased motor state variability in their day.63
A recent post-hoc analysis of the EASE LID and EASE LID 3 studies examined the effect of amantadine ER(Go) on activities of daily living, as measured by the UDysRS part 1B (N=196).16 At baseline, the majority of patients (63–73%) reported at least mild impairment of 6/10 UDysRS part 1B activities of daily living. Amantadine ER(Go) versus placebo significantly (p<0.05) reduced the impact of Parkinson’s disease symptoms on walking and balance, doing hobbies and other activities, public and social settings, exciting or emotional settings, in addition to eating tasks and speech. Furthermore, amantadine ER(Go) versus placebo significantly (p<0.05) reduced the clinician-rated intensity of dyskinesia in all seven of the body regions assessed in UDysRS part 3 and 3/4 clinician-assessed tasks assessed in UDysRS part 4. Finally, UDysRS part 1B, part 3, and part 4 total scores (LS mean difference: -3.9, -3.2 and -1.3 points, respectively) were also significantly improved (p<0.001) with amantadine ER(Go) versus placebo.
Patients with moderate-to-severe renal impairment (eGFR <50 mL/min/1.73 m2) were excluded from the clinical development programme for amantadine ER(Go).40,41,59 Renal impairment is more common in older patients who, in turn, may be more likely to have Parkinson’s disease,2,68 with one population estimate indicating that 20% of adults ≥65 years of age have at least moderate renal impairment.69 Consequently, an exposure simulation analysis of amantadine ER(Go) in patients with renal impairment was performed.70 Dose exposure was substantially higher in those with moderate to severe renal impairment than those without impairment. In particular, patients with moderate to severe impairment were estimated to have 3–5-fold higher maximum amantadine concentrations and 5–11-fold higher area under the curve with the 274 mg dose. In addition, simulations showed that patients with moderate and severe renal impairment took 1 week and 1 month, respectively, to reach steady-state concentrations. Consequently, physicians should consider the potential for age-related renal impairment and the need for lower doses when prescribing amantadine ER(Go), as per the FDA indication.36,70 In patients with renal impairment, the label recommends an initial dose of 68.5 mg once daily and a maximum maintenance dose of 137 mg once daily in patients with an eGFR of 30–59 mL/min/1.73 m2, and a maximum initial and maintenance dose of 68.5 mg once daily in those with an eGFR of 15–29 mL/min/1.73 m2. Note that amantadine ER(Go) is contraindicated in patients with an eGFR <15 mL/min/1.73 m2.36
The efficacy of amantadine ER(Go) does not appear to be affected by age, with similar reductions in time spent ON with dyskinesia and OFF across all age groups observed in a pooled analysis of the EASE LID and EASED LID 3 studies. In contrast, the incidence of AEs increased with age, with dizziness (23%) the most common AE in patients <65 years of age, hallucinations (32%) the most common in patients 65–74 years of age, and falls (27%) and hallucinations (27%) in patients ≥75 years of age.71 By comparison, in the overall population of patients (all age groups) receiving amantadine ER(Go), the incidence of hallucinations and falls was 21% and 13%, respectively, in the pooled analysis of the EASE LID and EASED LID 3 studies.61
Open-label results of amantadine extended-release Gocovri
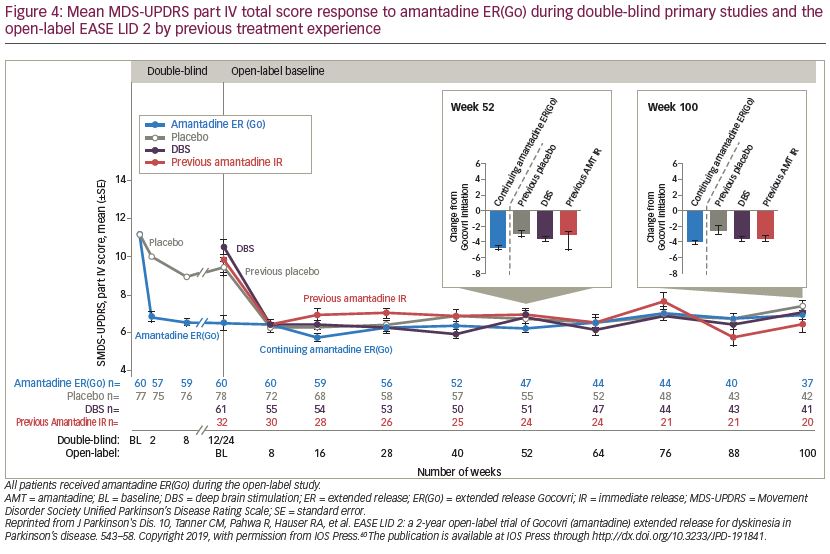
The 101-week open-label EASE LID 2 study (ClinicalTrials.gov Identifier: NCT02202551) assessed the long-term safety and durability of amantadine ER(Go).60 Patients who completed the EASED, EASE LID and EASE LID 3 studies, in addition to patients who were receiving DBS and therefore previously excluded from the phase II/III amantadine ER(Go) trials, were included. Patients were allocated to one of four protocol-defined subgroups based on their previous trial treatment assignment and whether they enrolled directly from a phase II/III study (1. continuing amantadine ER(Go); 2. previous placebo; and 3. participation gap groups) or were receiving DBS; 4. DBS group). All patients received amantadine ER(Go) 274 mg for weeks 2–100, with a lower (137 mg) dose given during the first (week 1) and last (week 101). Of the 223 patients enrolled, 75.8% completed year 1 of the study and 57.8% completed the full study (median treatment duration: 1.9 years). A total of 31 (13.9%) patients discontinued due to treatment-related AEs. The safety profile of amantadine ER(Go) was consistent with that observed in the randomized controlled phase II/III studies, with most AEs rated as mild to moderate in intensity (Table 1).40,41,59 Hallucinations were more common at the start of the study in patients naïve to amantadine ER(Go) compared with those continuing treatment (Table 1). As patients in the continuing-amantadine ER(Go) group had previously experienced a mean -4.7-point improvement from baseline in MDS-UPDRS part IV score in the phase II/III trials, EASE LID 2 baseline scores were lower (mean 6.5 points) than the three other subgroups (9.4–10.5 points). By week 8, all subgroups had similar scores (6.2–6.4 points) and improvements were maintained for the study duration (week 100 score: 5.6–7.0) (Figure 4).
One-year post-marketing pharmacovigilance
The 1-year post-launch safety profile of amantadine ER(Go), dispensed through a specialty pharmacy, which contacts patients by phone on a monthly basis, was consistent with that of the randomized, controlled studies.40,41,59,72 Subsequent to this analysis, the AE of seizure has been identified and is now included in the amantadine ER(Go) label, consistent with other amantadine formulations.36,39
Comparison of amantadine extended-release Gocovri, amantadine immediate-release and other therapies
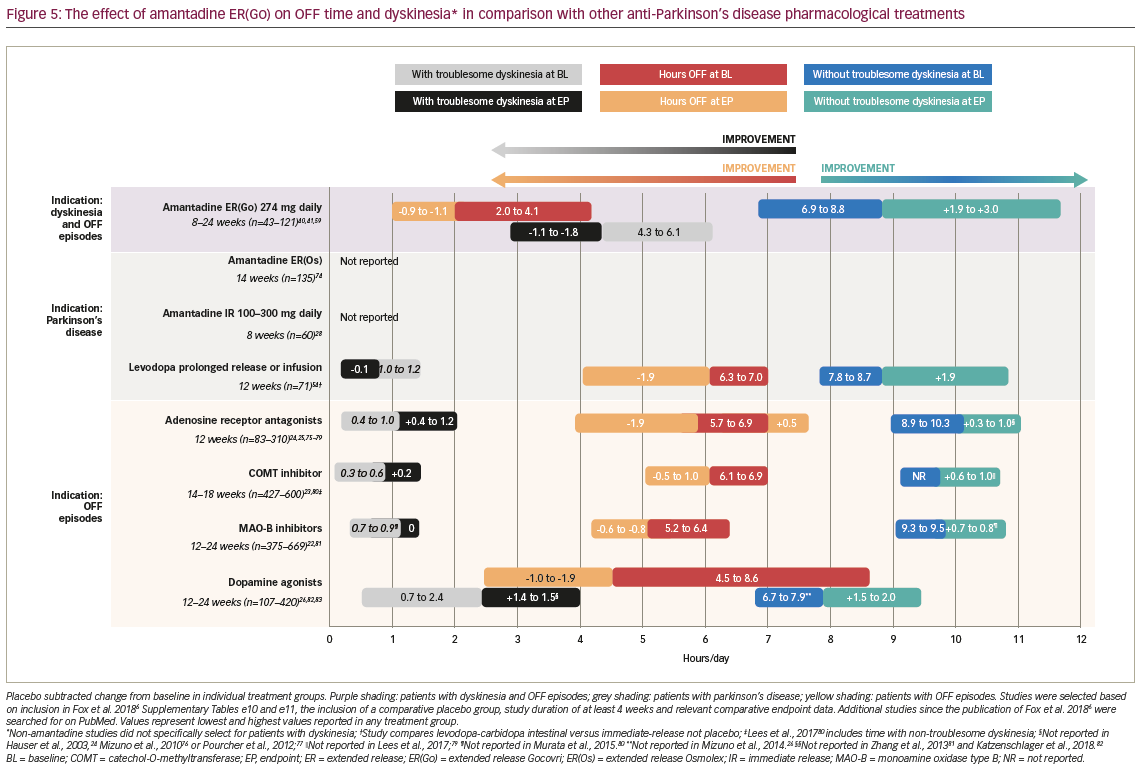
Several therapies have been examined for their potential to treat OFF episodes, including alternative levodopa formulations, adenosine receptor antagonists, COMT inhibitors, dopamine agonists and MAO-B inhibitors, in addition to the dopamine agonist pramipexole and amantadine IR for dyskinesia (Figure 5).22–26,28,41,54,59,73–82 Amantadine ER(Go) treatment has generally resulted in similar reductions in time spent OFF (~-1.0 hour/day) compared with other therapies investigated for treating OFF episodes (~0.5–2.0 hours/day improvement from baseline).22–26,28,41,55,59,73–82 However, for most of these therapies, improvements in good ON time without troublesome dyskinesia have been modest versus placebo, with less than an hour per day gained in comparison with the 1.9–3.0 hours per day reported for amantadine ER(Go) (Figure 5).22–26,28,41,55,59,73–82 Additionally, most treatments do not reduce, or may worsen, troublesome dyskinesia, whereas amantadine ER(Go) reduced this outcome by ~1.0–1.5 hours/day. With a unique formulation, it is the only amantadine product indicated for bedtime administration, and did not show apparent effects on asleep time in clinical trials.36,83 Other novel therapies that have been investigated for dyskinesia include glutamatergic, noradrenergic, histaminergic, serotonergic and cholinergic agents.84 However, the majority of these agents have not proven beneficial in clinical studies due to poor efficacy, safety concerns or a worsening of motor symptoms.84–87
To date, the efficacy and safety of amantadine ER(Go) have not been directly compared with amantadine IR in a head-to-head randomized clinical trial, information which would be clinically informative for physicians in deciding whether to try amantadine IR or amantadine ER(Go) first. However, the benefit of switching from amantadine IR to amantadine ER(Go) was also examined in a small subset (n=32) of patients in the EASE LID 2 study. Improvements in motor complications with amantadine ER(Go) were assessed in patients previously receiving amantadine IR (mean 2.5 years of exposure) at EASE LID 2 enrolment. The 24 patients previously treated with amantadine IR who completed week 52 demonstrated improvements from baseline in MDS-UPDRS part IV scores (-3.0), greater than the proposed minimal clinically important difference of 0.9 points;88 this benefit was maintained until week 100, with a similar safety profile to previous amantadine ER(Go) studies.40,41,59 Further data from a larger group of patients on the benefit of switching to amantadine ER(Go) from amantadine IR would be informative. It should also be noted that amantadine ER(Os), a combination of IR and ER, has not demonstrated consistent efficacy in treating dyskinesia and was approved on the basis of bioequivalence with amantadine IR.42 As described in the FDA-approved prescribing information, amantadine ER(Go) is not interchangeable with other amantadine products.36
The clinical development programme for amantadine ER(Go) had several important improvements over the studies investigating amantadine IR. One feature was the placebo-controlled, multi-arm, multicentre design of the studies. In contrast, as noted by a 2003 Cochrane review, many previous studies examining the efficacy and safety of amantadine IR for dyskinesia used withdrawal methodologies or cross-over designs without a washout period, potentially allowing for a carryover effect of treatment.30,32,34,58 In addition, efficacy and safety results for both amantadine and placebo groups have been frequently combined when the results of these studies have been presented.30,32 Furthermore, several previous studies have not randomly allocated patients to treatment, potentially biasing results.58 The EASED, EASE LID and EASE LID 3 studies were all 2–6 months in duration and used a gold-standard, dyskinesia rating scale, in addition to the 2-year open-label EASE 2 extension study being, to our knowledge, the longest assessment of amantadine for dyskinesia conducted.40,41,59,60
A further important difference between the amantadine ER(Go) and amantadine IR studies is the inclusion of a defined population of patients with daily troublesome dyskinesia. The majority of amantadine IR studies included a more general population of patients with Parkinson’s disease and did not include specific dyskinesia criteria, making treatment benefits more difficult to detect.28,40,41 The amantadine ER(Go) studies all included patients with evidence of troublesome dyskinesia impacting their daily living, the target population for amantadine ER(Go).40,41,59 The results may not be generalizable to effects of amantadine ER(Go) on PD symptoms in patients without dyskinesia, and do not evaluate whether amantadine ER(Go) would prevent dyskinesia in PD patients who do not yet have the condition.
Conclusion: Clinical significance of the development of amantadine extended-release Gocovri for dyskinesia and OFF
The development programme for amantadine ER(Go) can also be put in the context of recent controversies regarding the importance of addressing dyskinesia in Parkinson’s disease.89 An opinion article by Chaudhuri et al., questioned the need to treat dyskinesia following recent improvements in levodopa therapy, the number of patients who find dyskinesia troubling and the duration of dyskinesia.89 However, as discussed above and by an opinion article by Cenci et al., levodopa-sparing strategies may increase patient time spent OFF.47 Patient awareness of dyskinesia may be limited and worsened by dopaminergic overstimulation of mesocorticolimbic circuits, and many studies have demonstrated that dyskinesia has a negative impact on many aspects of HRQoL. Together, this suggests the results for amantadine ER(Go) may represent an important therapeutic alternative to current treatment strategies to improve functional or good ON by reducing both dyskinesia and OFF.
Dyskinesia and OFF episodes result in fragmentation of a patient’s waking day, and thus have a large impact on patient HRQoL and daily living in Parkinson’s disease. Dyskinesia also represents a challenge to continued dose escalation of levodopa to treat OFF episodes. Existing treatments (e.g. MAO-B inhibitors and COMT inhibitors) for OFF have demonstrated efficacy, but either worsen or cause dyskinesia. Treatment with amantadine IR has been reported to decrease dyskinesia, although with little or no sustained effect on OFF and poor tolerability at higher doses. Amantadine ER(Go), which has a delayed time to maximum concentration allowing for once-daily bedtime dosing and continuous coverage upon awakening in the morning and throughout most of the day, has been demonstrated in three randomized clinical studies to reduce dyskinesia and, in two studies, OFF time. The open-label, 2-year EASE LID 2 study has supported the durability of these effects and further analyses have suggested that amantadine ER(Go) improves activities of daily living and reduces the number of transitions between motor states. This results in less fragmentation of a patient’s day by allowing longer durations of ON time without troublesome dyskinesia or OFF episodes. Together, these results suggest that amantadine ER(Go) represents a clinically significant advance on existing treatments that target OFF episodes (but can increase dyskinesia) by improving both dyskinesia and OFF time.