While Alzheimer’s disease (AD), the most common cause of dementia, is perhaps best characterized by cognitive decline, more than 90% of patients with dementia exhibit behavioural and psychological symptoms of dementia.1 The Cache County Study on Memory in Aging describes six areas of neuropsychiatric behavioural abnormalities in patients with AD and other dementias, comprising aggression, apathy, depression, sleep, psychosis and psychomotor agitation.2 Agitation was noted to be positively associated with disinhibition, irritability and aberrant motor activity.2 While there is no consensus regarding the definition of agitation, the Agitation Definition Work Group of the International Psychogeriatric Association has defined it as having the following components: (1) occurs in patients with cognitive impairment or dementia; (2) patients exhibit behaviour consistent with emotional distress; (3) manifests with excessive motor activity or verbal/physical aggression; and (4) manifests with behaviours causing excess disability not solely attributable to another psychiatric, medical or substance-related disorder.3 Agitation is positively associated with disinhibition, irritability, delusions and aberrant motor activity, and has a significant negative effect on perceived quality of life in patients with dementia and on the quality of life of their caregivers.4 For example, agitation and irritability in patients with AD have been associated with increased morbidity and mortality, greater caregiver stress and earlier placement in long-term care facilities such as nursing homes.5 ‘Sundowning’, which refers to a state of confusion starting in the late afternoon and continuing into the night, is often of particular concern to patients and their caregivers, as it can be associated with aggression and agitation.6
While the neurochemical origins of dementia-related agitation (DRA) are unclear, it has been associated with decreased 5-hydroxytryptamine (5-HT2A) receptor binding and reduced 5-HT2A receptor density in the prefrontal cortex.7 In addition, neurodegeneration of noradrenergic neurons in the prefrontal cortex may account for agitation and aggression in AD.8
Evaluation and non-pharmacological management of
dementia-related agitation
The first-line treatment of DRA comprises non-pharmacological interventions, which are becoming increasingly favoured because of their lower risk of side effects compared with pharmacological treatment (Table 1).9–22
Causes of agitation and aggression
Before considering interventions addressing agitation caused by dementia, it is important to consider and address other causes of agitation and aggression. One such cause is delirium superimposed on dementia (DSD), which occurs when delirium develops in a person with pre-existing dementia.23 Although its prevalence ranges from 22% to 89% in hospitalized and community-based older adults (age 65 and over), DSD is unfortunately commonly unrecognized or misdiagnosed, with approximately half of DSD cases going unrecognized by healthcare personnel.24,25 There are several potential causes of DSD, including medications (e.g. anticholinergics, benzodiazepines), infections, electrolyte disturbances, alcohol or medication withdrawal, and hypo- or hyperglycaemia.26 It is therefore important to employ a systematic approach to identifying these possible causes and intervene accordingly.26 One first step might be review a patient’s medication lists and discontinue inappropriate drugs. Polypharmacy is prevalent among older adults, and such action can be helpful in managing behavioural symptoms in patients with dementia.27
Pain can also cause agitation and aggression in patients with dementia.27 A cluster randomized placebo-controlled trial of nursing home residents with moderate to severe dementia (n=352) found that treating pain for 8 weeks according to a stepwise protocol was associated with significantly reduced agitation compared with usual treatment, as measured using the Cohen-Mansfield Agitation Inventory (CMAI).28 With pain control, the average reduction in agitation was 17%; withdrawal of pain treatment worsened agitation.
Individualized interventions
When the patient’s agitation cannot be attributed to other addressable causes, DRA must be considered and appropriate non-pharmacological interventions provided. Perhaps some of the most promising evidence comes from a randomized controlled cluster trial comparing the effects of usual treatment versus the WHELD (Well-Being and Health for People with Dementia) programme on agitation in patients with dementia (n=847).9 The WHELD programme consisted of training care-home staff on person-centred care and social interaction, and educating them on antipsychotic medications (antipsychotic review). After 9 months, the WHELD intervention was associated with a statistically significant improvement in CMAI scores among patients. It was also shown to have a significant cost advantage for the general healthcare system compared with usual treatment.9
Another placebo-controlled trial involving 167 geriatric nursing home residents with dementia showed that individualized non-pharmacological interventions to treat agitation can be beneficial.29 Interventions were tailored to each patient’s unique cognitive, physical and sensory status, and their habits and roles. The implementation of these interventions resulted in statistically significant decreases in overall agitation relative to a control group, according to assessments based on the Agitated Behavior Mapping Instrument and Lawton’s Modified Behavior Stream.29
However, not all models have been shown to be effective in reducing DRA. Dementia Care Mapping (DCM) is often used with the aim of decreasing agitation in residents with dementia in long-term care. Through this model, care workers follow a formal process of continuous observation, evaluation and changes in the care of patients.10 However, a cluster randomized controlled trial investigating DCM’s effects on agitation in residents with dementia compared with placebo (n=726 at baseline and n=261 at 16 months) showed that DCM was not associated with statistically improved CMAI scores.10 In addition, the authors suggested that standard care homes may lack sufficient resources for sustained DCM, as only a quarter of homes completed more than one DCM cycle.
Other interventions
Although WHELD and other individualized non-pharmacological interventions to treat DRA have been shown to be effective, it is also important to be aware of others types of popular interventions, each of which has varying degrees of reported success. After identifying environmental and non-environmental triggers, the following patient interventions should be considered.
Structured activities have been shown, in some studies, to be beneficial to both patients and caregivers. A double-blind, randomized crossover study (n=133) found that, compared with control individuals, long-term care residents with dementia who engaged in Montessori-based activities 6 days a week for 4 weeks had significantly reduced agitated and aggressive behaviours, as measured by the CMAI.11 In addition, caregivers were given education and training and reported significantly improved ease-of-care ratings in the Montessori-based activity group compared with controls.11 This suggests that in-service training for caregivers in using interventions can be beneficial to both the caregivers and to patients. Another prospective, randomized, placebo-controlled study (n=60) investigated the effects of a tailored activity programme on patients with dementia who were living in their own home and their family caregivers.12 The programme was implemented monthly for 8 months and included instruction to caregivers regarding activities. The researchers found that, at 4 months, caregivers reported fewer problem behaviours and greater activity engagement from patients, and fewer caregivers reported patient agitation and argumentation. In terms of caregiver benefits, the researchers found that caregiver burden was reduced, with families reporting fewer hours being on duty, and that caregivers’ self-efficacy and skill sets were improved.12
Massage and other approaches using therapeutic touch may also be effective in reducing some manifestations of DRA. A multiple time series, blinded study of 51 long-term care facility residents with AD compared the effectiveness of therapeutic touch, simulated therapeutic touch and usual care on DRA and related disruptive behaviours.13 The study found that physical non-aggressive behaviours were significantly reduced in those who received therapeutic touch versus simulated touch and usual care. However, no significant differences were observed in physically aggressive or verbally agitated behaviours. In another randomized, double-blind, placebo-controlled study (n=57), nursing home residents experiencing behavioural symptoms of dementia who were given therapeutic touch had significant reductions in overall behavioural symptoms of dementia, manual manipulation and vocalization compared with residents receiving simulated and usual care.14 The treatment consisted of therapeutic touch given for 5–7 minutes twice daily (once in the morning and once in the afternoon) for 3 days.
Exercise and other physical activities may also be helpful in reducing DRA. A prospective comparative study (n=50) found that nursing home residents with dementia who participated in a 3-week exercise programme had improved Pittsburgh Agitation Scale (PAS) scores compared with residents who did not participate.15 The exercise programme consisted of 15 minutes of aerobic exercise and 15 minutes of resistance exercise 3 days per week. In addition, patients with a pre-exercise programme PAS score of higher than 3 (i.e. categorized as agitated) had greater decreases in agitation than those with lower initial PAS scores. Another randomized placebo-controlled trial (n=70) compared the effects of a 2-week exercise programme with a control social-stimulation programme in patients receiving acute hospital dementia care.16 The intervention consisted of 20-minute exercise sessions on 3 days/week. The study found that patients who participated in the exercise programme had significant improvements in the Alzheimer Disease Research Center–Clinical Global Impression of Change (CGIC) dimensions of emotional agitation, lability, psychomotor agitation and verbal aggression.
There is mixed evidence for music therapy in reducing DRA. For example, a systematic review of 180 studies found that of a variety of non-pharmacological interventions used to treat behavioural and psychological symptoms of dementia in elderly patients, music therapy was the only intervention that was associated with statistically significant reduction in agitation (n=397), apart from behavioural management.17 The therapy involved listening, moving or dancing, and/or singing or playing an instrument, and was sometimes administered alongside exercise and reminiscence therapy. However, a randomized controlled trial comparing the effects of music therapy with general activities in nursing home residents with dementia showed that while CMAI scores were significantly reduced with music therapy, there was no additional benefit of music therapy over general activities.18 Similarly, a systematic review of 160 papers showed that in addition to person-centred care and sensory therapy, structured music therapies may help to reduce mild-to-moderate agitation in the short term, but lack significant long-term benefits.19 In addition, they had no beneficial effects on severe agitation symptoms.19
Bright-light therapy and sleep improvement are also sometimes used to reduce DRA, but have shown limited efficacy in clinical trials. A randomized, controlled, crossover clinical trial (n=15) conducted in a chronic care facility with patients with dementia and agitated behaviours administered bright-light therapy for 1 hour/day for 4 weeks, after which patients had 1 week with no treatment before being crossed over to the control arm.20 The researchers found that while bright-light therapy led to more hours of sleep, it did not improve agitation (according to Behavioral Pathology in AD scores) in patients with non-disturbed sleep–wake cycles. Another randomized placebo-controlled trial (n=48) also showed that while sleep improved, there was limited evidence that bright-light therapy improved agitation.21 However, it was suggested that the treatment might have greater efficacy in the winter.
Simulated presence therapy, which involves playing video or audio recordings of family members to patients with dementia, has also been thought to be useful in improving DRA. However, a systematic review (n=144) of randomized and quasi-randomized controlled trials and crossover studies showed that there is inadequate evidence for the effectiveness of this intervention in patients with dementia and agitation, mainly because of differences in participants, the format of therapy, comparison interventions and outcome measures among the studies.22 Thus, more studies are necessary before simulated presence therapy can be recommended as an evidence-based treatment.
In summary, the current literature is ambiguous regarding the efficacy of non-pharmacological interventions for DRA. This is perhaps best illustrated by a systematic review and meta-analysis of 19 studies by Jutkowitz et al.30 The authors showed that there is insufficient evidence to support non-pharmacological interventions including staff training, care-delivery models and changes to the environment in improving agitation and aggression in nursing home and assisted-living residents, according to CMAI scores. The strength of evidence was also deemed to be generally insufficient to compare treatment efficacy.30
Current pharmacological management of dementia-related agitation
If non-pharmacological measures are unsuccessful in reducing DRA then medications should be considered (Table 2).31–40 Currently, there are no US Food and Drug Administration (FDA)-approved treatments for behavioural disturbances associated with dementia; as such, the following drugs are used off-label for this indication.41
Atypical antipsychotics
Some of the most widely used drugs for this purpose include atypical antipsychotics.41 The Clinical Antipsychotic Trials of Intervention Effectiveness–Alzheimer’s Disease (CATIE-AD) was a randomized, placebo-controlled trial investigating the effects of olanzapine, quetiapine and risperidone in patients with AD who had disruptive delusions, hallucinations, aggression or agitation that developed after the onset of dementia.31 The study (n=421) revealed that at 12 weeks, CGIC scores were improved with use of each antipsychotic, although the changes were not statistically significant versus placebo. Specifically, 32% of patients taking olanzapine, 29% of those taking risperidone, 26% of those taking quetiapine and 21% of those taking placebo had a CGIC score indicating at least minimal improvement. In addition, the study revealed that median time to discontinuation of treatment due to lack of efficacy favoured olanzapine and risperidone. In terms of adverse events, the proportion of patients experiencing extrapyramidal symptoms and sedation were not significantly different among the groups. Still, these adverse effects were deemed to offset any potential advantages in the efficacy of atypical antipsychotic drugs for treating agitation and other behavioural symptoms of AD.31
Other studies have corroborated these results. A systematic review of 16 meta-analyses by Tampi et al. found that atypical and typical antipsychotics are associated with modest efficacy in treating psychosis, aggression and agitation in patients with dementia, but that their use is often limited by their adverse effect profile.32 Given these findings and their black box warnings for elderly patients with dementia, antipsychotics should be used cautiously in the treatment of DRA.42 They can be given orally or parenterally, and should be used as first-line therapies in emergency situations to prevent harm to the patient or others.43
After unsuccessful treatment with non-pharmacological methods, healthcare providers should discuss the risks and benefits of pharmacological therapies with caregivers so that an informed decision can be made together. If the patient has symptoms of psychosis, the lowest maintenance dose of the preferred atypical antipsychotic should be determined before its administration.36
Antidepressants
While less commonly used than antipsychotic medications, antidepressants may be effective in reducing behavioural symptoms of dementia. A Cochrane meta-analysis (n=692) compared the effects of selective serotonin reuptake inhibitors (SSRIs) with placebo in elderly adults with dementia who had agitation and/or psychosis.33 The study revealed that there was a significant difference in measures of agitation (reported by CMAI scores) in patients taking SSRIs versus placebo, although the authors noted that the results were heavily weighted by one large study. However, there was no significant difference in change in behavioural symptoms between patients taking SSRIs versus placebo as measured using the Neuropsychiatric Inventory (NPI) and Behavioral Pathology in Dementia scales. The meta-analysis also showed that sertraline and citalopram were associated with a reduction in symptoms of agitation when compared with placebo. SSRIs and trazodone were determined to be tolerated reasonably well compared with placebo.33
Another meta-analysis by Hsu et al. compared the effects of SSRIs with placebo on overall neuropsychiatric symptoms and agitation in patients with dementia. The study showed that, compared with placebo, SSRIs significantly reduced overall neuropsychiatric symptoms and agitation severity, as well as caregiver burden.34
Anticonvulsants
Anticonvulsants are sometimes used to improve DRA. Of these drugs, carbamazepine has the most evidence supporting its use. A randomized, multisite, double-blind, placebo-controlled study (n=51) found that, in nursing home patients with agitation and dementia, carbamazepine led to a 77% global improvement in Clinical Global Impression- Improvement (CGI-I) scores, compared with 21% in patients taking placebo.35 Brief Psychiatric Rating Scale (BPRS) scores were also significantly improved. Secondary analyses by the authors confirmed that these scores were due to decreased agitation and aggression. The drug was taken for 6 weeks with a mean serum level of 5.3 μg/mL. Although patients showed no changes in functional status or cognition, side effects were more common in patients taking carbamazepine (59%) than in those taking placebo (29%). Two events in the treatment group were considered clinically significant, with one patient experiencing tics and the other developing ataxia. In terms of caregiver changes, staff perceived they spent less time managing agitation for patients taking carbamazepine but not placebo.35 In addition to this study, a meta-analysis of randomized placebo-controlled trials showed significant improvements in BPRS and Clinical Global Improvement- Severity(CGIS) scores with carbamazepine compared with placebo in patients with AD and dementia, implying preliminary evidence for carbamazepine as an anti-agitant in dementia.36
In terms of other anticonvulsants, a meta-analysis showed no improvement in agitation among patients with dementia taking valproate compared with controls. In addition, there was an increase in adverse events including falls, infections and gastrointestinal issues.45 More research is needed on the use of newer anticonvulsants before they can be recommended.
N-methyl-d-aspartate receptor antagonists
Memantine, an N-methyl-d-aspartate (NMDA) receptor antagonist, is another drug that has been studied for its efficacy in reducing DRA, although it has not been shown to be effective. One double-blind randomized controlled trial (n=149) investigated memantine’s effect compared with placebo on clinically significant agitation in patients with AD in care homes and hospitals, and found no significant difference in CMAI scores between the two groups after 6 or 12 weeks.37 In addition, a Cochrane meta-analysis of double-blind, parallel group, placebo-controlled randomized trials (n=422) investigating the effects of memantine in people with dementia concluded that there is moderate‐certainty evidence from two studies to suggest there is probably no difference in CMAI scores between memantine and placebo; the relative improvement was 0.50 CMAI points (95% confidence interval –3.71 to 4.71), which was deemed a very small change.38
Benzodiazepines
Benzodiazepines are occasionally used to reduce DRA but – due to a lack of evidence supporting their efficacy in the long term as well as their potential for severe adverse effects – they should be avoided, especially in long-term use. Evidence supporting the efficacy of benzodiazepines comes from a multicentre, randomized, double-blind, placebo-controlled study (n=272) that was conducted in acutely agitated patients with AD and vascular-related dementia.39 Patients in the treatment groups received either intramuscular olanzapine (2.5 or 5.0 mg) or lorazepam (1.0 mg). The study showed that, at 2 hours, both doses of olanzapine and lorazepam were associated with significant improvements compared with placebo in scores on the PANSS Excited Component (PANSS-EC) and the Agitation–Calmness Evaluation Scale. All treatment groups also showed more improvement in CMAI scores versus placebo. At 24 hours, both olanzapine (5.0 mg) and lorazepam were associated with improved Agitation–Calmness Evaluation Scale scores compared with placebo, while only olanzapine (both doses) improved PANSS-EC scores; Mini-Mental State Examination scores were not significantly improved in any group. Adverse events were not significantly different for any group compared with placebo.39 However, other studies have reported no significant effect of benzodiazepines in the long term. For example, a randomized, double-blind study (n=40) comparing the effects of alprazolam versus lorazepam in agitated patients with dementia found no significant improvement in agitation in patients taking lorazepam or alprazolam after 8 weeks.40
Any therapeutic effect of benzodiazepines in DRA may be secondary to anxiolytic and even sedating effects. Their prescription should be considered carefully, as they have been associated with increased confusion, impaired balance and an increased risk of falls.40,46 Agitation may also be a side effect of benzodiazepines.40 However, this may be due to psychotropic polypharmacy, as one study showed that exclusive use of either antipsychotics or benzodiazepines was not significantly associated with worsening cognitive and neuropsychiatric symptoms in patients with dementia.47
Emerging pharmacological treatments for dementia-related agitation
Brexpiprazole
Several drugs are being investigated for a potential role in improving DRA (Table 3). One of these is brexpiprazole, an atypical antipsychotic that was approved by the FDA in 2015 for the treatment of schizophrenia and augmentation in major depressive disorder.48 Brexpiprazole antagonizes 5-HT2A receptors and, to a lesser extent, partially agonizes the dopaimine-2 receptor, which lowers the risk of extrapyramidal side effects.49 Although the drug is still under investigation for a role in DRA, initial findings have been promising.49 A randomized, double-blind, placebo-controlled, parallel-arm study in patients with AD and agitation found that 2 mg/day brexpiprazole was associated with statistically significant improvements in CMAI total score after 12 weeks compared with placebo.50 The drug was also well tolerated; the most common treatment-emergent adverse events were mild or moderate in severity, with headaches being most frequent.
Dextromethorphan combinations
Another pipeline drug is AXS-05, which is a combination formula of dextromethorphan and a small proportion of bupropion.49 Dextromethorphan is an FDA-approved antitussive that acts on several receptors, including sigma-1, serotonin transporter, noradrenaline transporter, NMDA, α-2 adrenoreceptor, the H1 receptor and nicotinic α3β4 receptors.51 Murine models have suggested that dextromethorphan may also be implicated in sigma-1-dependent stimulation of the dopaminergic system, which makes it an attractive candidate for modulating agitation.52 On the other hand, bupropion inhibits noradrenaline and dopamine reuptake.53 It is a cytochrome P450 2D6 inhibitor, and was therefore added to the formula to reduce dextromethorphan’s first-pass effect in the liver and prolong its plasma half-life.53 AXS-05 has been fast-tracked by the FDA to evaluate its effectiveness for reducing agitation in patients with AD.54 An Axsome Therapeutics phase III, double-blind, placebo-controlled clinical trial (n=435) is currently under way (ClinicalTrials.gov identifier NCT04947553). The interim analysis showed that one tablet of AXS-05 per day for 5 weeks was associated with improved CMAI scores.55
Another drug being researched for DRA is AVP-786, which is a combination formula of deuterated dextromethorphan and quinidine, the latter of which is an antiarrhythmic drug.49 Like bupropion, quinidine inhibits cytochrome P540 2D6 and is added to prolong dextromethorphan’s plasma half-life. Deuteration of dextromethorphan also improves the drug’s first-pass metabolism.56
Prior to the development of AVP-786 AVP-923 (Nuedexta®; Avanir Pharmaceuticals, Aliso Viejo, CA, USA) was shown to be a promising candidate for DRA treatment.49 However, its development was stalled due to AVP-786’s comparative ability to slow liver metabolism and prolong its half-life.58 Approved by the FDA for pseudobulbar affect, AVP-923’s drug formula consists of non-deuterated dextromethorphan and quinidine.57 In a phase II randomized, multicentre, double-blind, placebo-controlled trial (n=220), agitated patients with AD were given AVP-923 20/10 mg once daily, increasing to 30/10 mg twice daily, for 10 weeks.59 AVP-923 administration resulted in statistically significant improvements in NPI agitation/aggression domain scores compared with placebo.59 Following these encouraging results, in 2015 the manufacturer initiated a phase III clinical trial to evaluate the efficacy and long-term safety of AVP-786 for DRA.60
It is important to consider that AVP-923 was given a black box warning for patients with congenital long-QT syndrome, and so AVP-786 will likely also have to be avoided in these patients.58 In addition, the QTc interval should be periodically measured in patients who take the drug and are at risk of QTc prolongation.61
Cannabinoid receptor agonists
Cannabinoid receptor agonists such as tetrahydrocannabinol (THC), dronabinol (Marinol) (currently FDA approved as an appetite stimulant) and nabilone have also been studied for treating DRA.49 One reason for this is that, at non-psychoactive doses, activating cannabinoid receptors can influence mood and behaviour.62 However, clinical trials have suggested mixed results. A randomized, double-blind, placebo-controlled study (n=50) found that in patients with dementia experiencing agitation or aggression, 1.5 mg THC for 3 weeks was not significantly associated with improved NPI scores compared with placebo.63 Similarly, another randomized, double-blind, placebo-controlled, repeated crossover study (n=22) in patients with dementia with neuropsychiatric symptoms did not show significantly improved NPI scores in a THC group when compared with placebo.64 On the other hand, studies evaluating dronabinol have been more promising.49 A retrospective study (n=40) showed that in patients with severe dementia, 7 mg dronabinol for 2 weeks was associated with significant decreases in all domains of the PAS.65 However, adverse effects including sedation have been associated with the drug.65
The efficacy of nabilone is currently being investigated. A randomized, double-blind, placebo-controlled crossover trial (n=39) showed that 1–2 mg/day nabilone for 14 weeks statistically improved CMAI scores and agitation in patients with AD with agitation compared with placebo.66 Participants did experience sedation, and larger studies are needed to further evaluate nabilone’s side effects and therapeutic efficacy.
Pimavanserin
Another promising drug is pimavanserin (Nuplazid®; Acadia Pharmaceuticals Inc., San Diego, CA, USA), which the FDA approved in 2016 for treating psychosis in patients with Parkinson’s disease.67 The drug is a 5-HT2A receptor inverse agonist without affinity for dopaminergic receptors.49 As such, it does not cause extrapyramidal side effects, which is particularly important in geriatric patients.49 The manufacturer is currently conducting a phase III trial (n=750) to investigate the efficacy of pimavanserin in treating psychotic symptoms and agitation in patients with dementia (EudraCT number: 2017-004439-36).68
BXCL501
Another candidate for treating DRA is sublingual oral dexmedetomidine (BXCL501), which is a selective α-2a adrenergic receptor agonist.69 It is currently used in its FDA-approved IV form (Precedex®; Hospira, Lake Forest, IL, USA) in intensive care units for its anxiolytic and sedative effects.70 In 2021, the FDA granted the drug breakthrough therapy designation for the acute treatment of agitation in patients with dementia.71 A phase III randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov identifier NCT05276830) has been initiated after a phase Ib/II randomized, double-blind, placebo-controlled trial showed significant improvements in agitation in patients with dementia taking 30 and 60 μg BXCL501.72
Prazosin
Prazosin, an α-1 adrenoreceptor inhibitor, may also have potential in treating DRA.73 It is the only drug in its class that crosses the blood–brain barrier to act directly on the central nervous system.73 A small, randomized, double-blind, placebo-controlled study (n=22) showed that a mean dose of 5.7 mg/day for 8 weeks significantly improved dementia-related aggression and agitation per BPRS and NPI scores compared with placebo.74 There was no significant difference between the two groups in side effects. A larger randomized, double-blind, placebo-controlled phase II study (n=186) is currently investigating the efficacy of prazosin (4–6 mg/day) in improving agitation, as measured by the PAS, PANSS-EC and modified CMAI scales, in patients with AD (ClinicalTrials.gov Identifier: NCT03710642).75
Electroconvulsive therapy for dementia-related agitation
For severe agitation and aggression that has not responded to non-pharmacological and pharmacological therapies, electroconvulsive therapy (ECT) may be an effective intervention, though more controlled studies would be useful. A systematic review showed that of 122 patients with dementia, 88% showed clinically significant improvements with ECT.76 Improvements often occurred early in the treatment course, and adverse events were mostly mild, transient or not reported. A prospective study in 23 patients with dementia who were referred for ECT to treat agitation and/or aggression reported significant decreases in agitation/aggression from baseline based on CMAI and NPI scores.77 Although treatment was well tolerated by most patients, it was discontinued for two patients because agitation recurred and in three patients due to adverse events. Further prospective studies are now needed to further investigate the potential role of ECT in reducing agitation in patients with dementia.76
Conclusion
Agitation in dementia is frequently difficult to manage, and is associated with increased morbidity and mortality in patients and heightened caregiver burden.3,5 A suggested approach to agitation is delineated by the mnemonic ‘DEMENTIA’ (Table 4).78
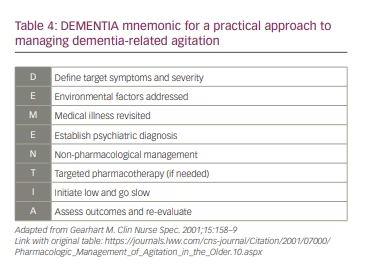
- The target symptoms and their severity must be Defined.
- Environmental factors should be identified and addressed.
- Care must be taken to consider and address other Medical illnesses that may be causing agitation.
- If other medical illnesses are excluded and criteria are met, an appropriate psychiatric diagnosis must be Established.
- Non-pharmacological treatment should be attempted first.
- If there is no response, Targeted pharmacological therapy can be employed after careful consideration, including of comorbidities and polypharmacy.
- Medications must always be Initiated at a low dose and slowly titrated to higher doses if needed.
- Outcomes should be Assessed and regularly evaluated.
For agitation refractory to non-pharmacological and pharmacological treatment, initial studies show that ECT may be an effective therapy, although prospective studies are needed before it can be recommended. While more robust randomized clinical trials are also awaited for other treatments, current strategies for managing agitation should be used to ensure holistic care for patients with dementia and their caregivers.