Neuromodulation is a recent technique that has increasingly been used for the abortive and preventive treatment of migraine and other primary headache disorders.1 It has previously been found to be helpful in the treatment of other neurological illnesses, such as epilepsy.2 Neuromodulation treatment of headache disorders involves pain relief via manipulation of the peripheral or central pain pathways with magnetic or electrical impulses.1 Currently, the use of neuromodulation in clinical practice remains limited. However, it can be an effective strategy either alone or in conjunction with other modes of therapy for migraine, particularly for patients who have had suboptimal response to, or intolerability and/or safety issues with, traditional pharmacotherapy.2,3
This article reviews neuromodulation techniques used for treatment of primary headache disorders, with a focus on migraine and cluster headache (CH). We explore eight invasive and non-invasive techniques: occipital nerve stimulation (ONS); trigeminal nerve stimulation; combined occipital and trigeminal nerve stimulation (COT-NS); sphenopalatine ganglion stimulation (SGS); caloric vestibular stimulation (CVS); remote electrical neuromodulation (REN); vagus nerve stimulation (VNS); and transcranial magnetic stimulation (TMS). We will review their mechanisms of action, expected physiological effects and the existing data on effectiveness.
Occipital nerve stimulation
ONS is an invasive neuromodulation technique that was described as early as 1977 by Picaza et al.,4 and has since been studied for use in a range of headache disorders.5–8 The therapy involves subcutaneous implantation of a small electrode at the level of the occipitocervical junction.9 The electrode can be implanted unilaterally or bilaterally, and it can be removed when needed.10 Once the electrodes are properly implanted, the patient can control delivery of electrical stimulation using a magnetic or wireless device in response to their symptoms. Adjustments can also be made by physicians at clinic visits. The electrical stimulation typically causes mild local paraesthesia in the sensory distribution of the occipital nerves; this induction of local paraesthesia is necessary to bring about the therapeutic effect of ONS. However, this has posed methodological challenges in research as it precludes evaluation of efficacy through masked placebo-controlled studies.10 Therefore, results of studies examining the efficacy of ONS must be interpreted with caution.
The exact mechanism through which ONS exerts its antinociceptive effect remains unknown. At present, ONS is thought to modulate the thalamic processing of nociceptive signals relayed by the trigeminocervical complex.11 Studies using metabolic neuroimaging have suggested that ONS induces slow neuromodulatory effects within the central nervous system, possibly helping to restore proper functioning of the pain control centres.12 If true, central modulation of the nociceptive afferent system could explain why ONS seems to improve a range of headache disorders.13 ONS may also exert an antinociceptive effect within the peripheral nervous system, for instance by modulating excitability of the peripheral nerve fibres.9,14
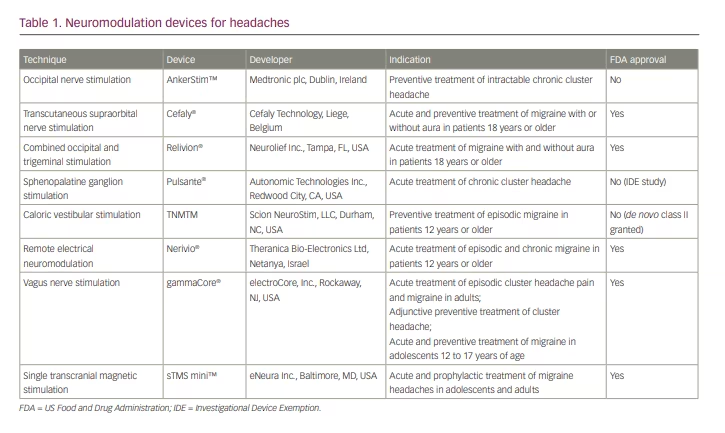
In recent years, ONS has been studied extensively in medically intractable CH. Two trials have reported the effectiveness of ONS in this population;12,15 however, both studies were limited by small sample sizes and lack of control groups. More recently, in 2021, a larger randomized, double-blind controlled study compared the effects of high versus low electrical dose of ONS in 131 patients with medically intractable CH.6 The study showed that approximately half of the subjects in both groups experienced a 50% reduction in attack frequency, suggesting that ONS, even at low stimulation intensity, is effective at preventing CH attacks in highly disabled patients who have not responded to conventional therapies.6 In fact, it was found that increasing the electrical dose does not seem to improve effectiveness, whereas it does increase patient discomfort. Research is ongoing to determine the optimal stimulation parameters for this patient population. At present, an ONS device called AnkerStim™ (Medtronic plc, Dublin, Ireland),16 though not approved by the US Food and Drug Administration (FDA), is currently available for the preventive treatment of intractable CH in the USA (Table 1).
The role of ONS in the treatment of chronic migraine is not as clear. Initially, retrospective studies suggested that ONS reduced headache frequency and severity.11,17,18 However, randomized controlled studies have since shown limited data. In 2010, a randomized controlled feasibility study involving 110 individuals with intractable chronic migraine showed that 39% of patients who received ONS with the ability to adjust stimulation dose reported greater than 50% reduction in headache days per month or a greater than 3-point drop in pain intensity from baseline.5 This was reportedly comparable to the benefit seen with conventional migraine preventive medications. However, this was a feasibility study and thus was not powered for efficacy evaluation.5 Systematic reviews and meta-analyses have been conducted in recent years to examine the efficacy and safety of ONS in chronic migraine.19,20 These reviews have highlighted considerable variability in the methods of pain assessment across studies and risk of bias in the assessment of efficacy. In particular, the effectiveness of blinding in randomized controlled trials (RCTs) remains unclear, casting doubt on the reliability of the reported findings. A pooled analysis of the effectiveness of ONS in reducing headache days and/or pain intensity did not show a statistically significant benefit.20
Given that ONS implantation is done surgically, there are possible complications. Some of these include surgical site infection and lead migration, which may necessitate additional procedures or treatment. Systematic reviews have indicated that these complications are relatively common, especially as the length of follow-up increases. However, long-term complications, including nerve damage, have not been reported.19,20
Although the invasive nature of ONS poses methodological challenges for research and potentially makes it a less preferable treatment modality to other non-invasive neuromodulation techniques, it remains an important treatment option for patients who suffer from medically intractable CH.
Trigeminal nerve stimulation
External trigeminal nerve stimulation, by way of transcutaneous supraorbital nerve stimulation (t-SNS), is a non-invasive technique that involves placement of adhesive surface electrodes that transmit electrical impulses on the forehead to stimulate the supraorbital nerves.2 A t-SNS device has been marketed as Cefaly® (Cefaly Technology, Liege, Belgium)21 since 2008, and it was approved by the FDA for migraine prevention in 2014 and subsequently for acute treatment of migraine with or without aura in 2017.22
To date, the strongest evidence for effectiveness of t-SNS in migraine prevention comes from the PREMICE study, a multicentre, randomized, sham-controlled trial conducted in Belgium from 2009 to 2011.23 The study demonstrated a reduction of 2 headache days per month on average in patients with episodic migraine who underwent daily, 20-minute sessions of t-SNS. No improvement was observed in the sham group. An updated analysis of the trial data indicated that the preventive effect of the device may be more pronounced in patients with more frequent migraine attacks.24
The device was subsequently evaluated in patients with chronic migraine in three small prospective open-label studies.25–27 In a 4-month pilot study that examined 23 patients with chronic migraine with and without medication overuse,8 patients experienced greater than 50% reduction in headache days per month.27 Over half of the patients were able to halve the use of abortive medications. Vikelis et al.26 evaluated the device in patients with episodic or chronic migraine in whom preventive therapy with topiramate had failed. This study showed a significant reduction in headache days, severity and use of abortive medications after a 3-month trial. The most recent open-label trial in 24 patients with chronic migraine showed a similarly statistically significant reduction in mean monthly headache days by 2.75.25 In all these studies, the device was well tolerated with no serious adverse effects. In addition, a survey study of 2,313 patients showed that the majority of users were satisfied with the benefit of t-SNS and were willing to purchase the device at the end of the 40-day trial period.28 A minority of patients (4.3%) reported adverse effects, including local pain, intolerance to paraesthesia, sleepiness, fatigue or insomnia, none of which was considered to be serious.
More recently, t-SNS has been evaluated for use in the acute treatment of migraine. In an open-labelled study, Chou et al.29 demonstrated significant reductions in pain severity and use of abortive medication after a 60-minute application of t-SNS during migraine attacks in 30 patients with episodic or chronic migraine. A retrospective study of 19 patients with vestibular migraine also reported improvement in both vertigo and headache severity with a 20-minute application of t-SNS.30
Similarly to other neuromodulation devices, the mechanism by which stimulation of the trigeminal nerve prevents and treats chronic migraine is not fully understood. It is speculated that peripheral neural stimulation may inhibit second-order pain receptors in the spinal trigeminal nucleus, while investigators believe that these devices also modulate the central nociceptive pathway in the brain based on functional brain imaging studies.31
The latest model of the Cefaly device is equipped with two settings to allow for both acute treatment of migraine attacks (single, 1-hour session during attack) and for prevention (daily, 20-minute session). In general, lack of insurance coverage has limited patients’ access to neuromodulation devices. However, the Cefaly device is currently covered by the US Department of Veterans Affairs, making it possible for wider use among veterans.32
Combined occipital and trigeminal nerve stimulation
COT-NS is a technique that seeks to modulate pain by concomitant stimulation of the occipital and trigeminal nerves. This technique was first investigated by Reed et al.33 in a case series of seven patients with severe chronic migraine. The technique was developed based on the observation that not all patients with migraine were responsive to ONS, as well as on the hypothesis that the trigeminal and occipital nociceptive afferents converge and share a final common pathway in the trigeminocervical complex. The case series demonstrated better pain control with the combined neurostimulation compared with ONS alone. Similar benefit of COT-NS was noted for hemiplegic migraine in a case series of four patients.34
In a randomized, sham-controlled, double-blinded study of 55 patients with episodic or chronic migraine,35,36 an external COT-NS device/headset was investigated with regards to its effectiveness in aborting migraine headaches.37 The headache relief rate, greater than 50% reduction in pain, and proportion of patients who reported no pain at the 2-hour mark were all significantly higher in the active treatment group compared with the sham treatment group. No serious adverse events were reported in this study.
The multicentre RIME study examined the effects of 1-hour self-administration of a COT-NS device in 131 patients with episodic migraine.38 Significantly more patients achieved complete freedom of most bothersome symptom (i.e. photophobia, phonophobia, nausea) within 2 hours of treatment. Significantly higher pain relief was observed in the active treatment group at 2 hours after treatment. Based on the results of the RIME study, Relivion® (Neurolief Inc., Tampa, FL, USA) obtained FDA approval in 2021 for acute treatment of migraine with or without aura in adults.39
Sphenopalatine ganglion stimulation
SGS is an invasive trigeminal nerve stimulation technique with the strongest evidence for use in acute and preventive treatment of CH. The sphenopalatine ganglion (SPG) is a large extracranial parasympathetic ganglion located on each side of the mid face within the pterygopalatine fossa. It contains multiple neural roots, including autonomic, sensory and motor, and is believed to play a role in headache and cranial autonomic symptoms associated with CH.40 In CH, the parasympathetic superior salivatory nucleus and SPG parasympathetic fibres are activated, inducing the release of neuropeptides and vasodilation of the cerebral and dural blood vessels. This process is thought to activate meningeal nociceptive fibres projecting to the trigeminal ganglion and nuclei, inducing referred pain in the periorbital area. This hypothesis led to the theory that blockage of the SPG may interfere with the pathological process and treat CH attacks.41
Several destructive procedures on the SPG, such as ganglionectomy, radiofrequency lesion, blocks and stereotactic radiosurgery, only showed a partial treatment benefit for chronic CH.41 Following this, a new non-destructive procedure targeting the SPG was developed. A chronically implantable neuromodulation device has been developed for acute SPG stimulation to abort the CH attacks on demand.42 The neurostimulator device is implanted in the pterygopalatine fossa with the lead in contact with the SPG. The device does not contain a battery but is activated and powered by remote control using radiofrequency energy.41
In a pilot placebo-controlled study of 28 patients with refractory chronic CH, alleviation of pain was achieved in 67.1% versus 7% of sham stimulation treated attacks (p<0.0001).43 Long-term results (24 months; 33 patients) confirmed the efficacy of SPG stimulation as an abortive treatment for acute CH attacks. Thirty-five per cent of patients also observed a >50% reduction in attack frequency, which suggested that SPG stimulation might also act as a CH-preventive treatment.44
The safety of SPG implantation procedure has been evaluated in a cohort study of 99 CH patients.45 Facial sensory disturbances were observed in 67% of the patients (46% of them being transient), transient allodynia in 3% and infection in 5%. Most (92%) of the adverse events were transient and evaluated as mild or moderate.45 This suggested that SPG stimulation may be an efficient and safe therapeutic technique that can be used for acute and preventive treatment of CH.
The Pulsante® (Autonomic Technologies Inc., Redwood City, CA, USA) device is under an investigational device exemption (IDE) study in the USA for approval by the FDA for the treatment of chronic CH;42 it is Conformité Européenne (CE) marked in Europe for the acute treatment of CH.46 There is increasing evidence that shows the effect of SGS on the autonomic and cerebral circulation, which potentially justifies a wider use of SGS for other chronic headaches and vascular diseases.47 More SGS studies in different headache disorders other than CH will be needed. In 2017, Pulsante received an expanded CE-marked indication to include pain relief for highly disabled migraine patients in Europe.46
Caloric vestibular stimulation
CVS has previously been used as a clinical tool to diagnose balance disorders and to confirm the absence of brainstem functions. By warming or cooling the external auditory canal with water or air irrigators, CVS creates convection currents in the endolymphatic fluid of the horizontal semi-circular canal. These currents change the firing rate of the vestibular nerves resulting in broad autonomic responses, including the vestibulo-ocular reflex.48
Although CVS has been a safe tool, the exploration of its therapeutic potential in other areas has been interrupted because of the unpleasant side effects, thermal imprecision, lack of dose control and administration complexity.49 However, these obstacles have recently been overcome with the development of a solid-state device for delivering CVS.50
The potential for CVS as an effective prophylactic treatment for episodic migraine has been noted in several studies. Various neuroimaging studies suggest that there is involvement of brainstem dysfunction in migraine.51–53 Recent transcranial Doppler sonography data have also shown that CVS treatment generates changes in cerebrovascular dynamics, which suggests brainstem modulation.50 The implied mechanism of action of CVS is that it activates vestibular nuclei in the brainstem, which sends both direct and indirect excitatory afferents to various brainstem nuclei. These vestibular nuclei have an extensive polysynaptic connection with many midbrain and forebrain structures that are involved in migraine pathology.48 In addition, although small and uncontrolled, a few studies have shown that caloric stimulation helped acutely alleviate various pain syndromes, including acute migraine.54–56
Based on these findings, a randomized, double-blinded, placebo-controlled trial was conducted to evaluate efficacy of CVS in prevention of episodic migraine.48 One hundred individuals were divided into a CVS treatment group and a placebo group, and completed a 3-month treatment regimen.57 CVS treatment was self-administered by wearing a CVS headset while lying supine on a 22° incline wedge pillow. The results showed a significantly reduced number of migraine days per month, subjective headache pain scores and the need for migraine abortive prescription medications by CVS treatment. There were no serious or unexpected adverse effects. This study suggested that CVS may provide a clinically efficacious and highly tolerable adjuvant therapy for the prevention of episodic migraine. TNMTM (Scion NeuroStim, LLC, Durham, NC, USA) was granted FDA de novo classification as a new device type in 2018 for prophylactic treatment of episodic migraine in patients 12 years or older. TNM received FDA class II approval, for which special controls are necessary to provide assurance of safety and effectiveness58 (Table 1).
Remote electrical neuromodulation
REN is a novel neuromodulation method for the acute treatment of migraine. It is thought to work through a mechanism referred to as conditioned pain modulation of upper arm peripheral nerves (median and musculocutaneous). Conditioned pain modulation is a descending endogenous generalized analgesic mechanism where pain in remote body regions is inhibited by subthreshold stimulation.59 REN is believed to activate descending analgesic pathways from the periaqueductal grey and the rostral ventromedial medulla in the brainstem and induce serotonin and noradrenaline release. Serotonin and noradrenaline are presumed to inhibit pain signals in the trigeminal cervical complex, which is activated during an acute migraine attack.60
The REN device is applied to the lateral upper arm between the bellies of the lateral deltoid and the triceps muscles to stimulate small skin nerves for 45 minutes. It is a wireless, battery-operated stimulation unit that is controlled by a smartphone application. It is used as an acute treatment during a migraine attack and can be used as a replacement or in addition to oral acute treatments for migraine. REN has been shown to have high tolerability with low adverse effect profile, mainly consisting of a transient sensation of mild warmth, redness or numbness of the arm/hand.60
The initial study was a prospective, double-blinded, randomized, crossover, sham-controlled trial with 86 patients who had migraine with or without aura. The 2017 study by Yarnitsky et al.61 demonstrated that early treatment with REN can significantly reduce acute migraine headaches. It showed a significant pain reduction in treatment within the first 20 minutes from the onset, compared with delayed treatment more than 60 minutes after the onset.
Then in 2019, Yarnitsky et al.62 published a pivotal randomized, double-blinded, sham-controlled and multicentre study that assessed the safety and efficacy of REN for the acute treatment of migraine. A total of 252 adult patients with migraine were randomized in a 1:1 ratio into active or sham stimulation groups. Participants treated their migraine within 1 hour of onset over 4 to 6 weeks with an electrical stimulation on the upper arm. The results showed a statistically significant benefit of REN over sham treatment in achieving pain relief, pain freedom and relief from the most bothersome symptoms at 2 hours. The clinical benefits were maintained for 48 hours post-treatment and in subsequent attacks. REN also demonstrated a favourable safety profile with a small number of adverse events, which was similar to the sham group.62
Another open-label, single-arm, dual-centre study by Nierenburg et al.63 in 2020 also demonstrated REN as a potential safe and effective non-pharmacological alternative for chronic migraine. It reported significant pain relief at 2 hours and 24 hours with REN treatment, with low device-related adverse events.
These studies suggest that REN may be a safe and effective alternative for treatment of acute migraine. The Nerivio® (Theranica Bio-Electronics Ltd, Netanya, Israel) REN device was approved by the FDA in May 2019 for acute treatment of migraine with or without aura in October 2020 for patients 18 years and older.64 Its approval was expanded in October 2020 and January 2021 for the acute treatment of patients aged 12 years and older with episodic and chronic migraine.64 This device is the first prescription smartphone-controlled wearable device for the acute treatment of migraine.
Vagus nerve stimulation
VNS is a type of neuromodulation that has been used for refractory treatment of depression and epilepsy. Its use in migraine was first reported in patients treated for seizures, whose headaches improved after weeks of implantation and use of a VNS device.65 The non-invasive VNS (nVNS) device stimulates the cervical branch of the vagus nerve through transcutaneous stimulation over the sternocleidomastoid muscle.66–69 It is a type of user-controlled stimulation that produces a low-voltage signal that delivers a current, with sessions lasting for 120 seconds and used in clinical trials up to 6 to 12 times per day.69
The mechanism of action of nVNS has been extensively studied in both animal models and humans, with evidence of anti-inflammatory effects and modulation of the trigeminal system.70 The main hypothesis is that the different connections of the trigeminal and vagus nerves in the brainstem are involved in the headache pain pathway.70 The trigeminal autonomic reflex, a physiological reflex that starts in the trigeminal nerve, projects towards the trigeminal cervical complex in the brainstem and then to superior brain structures, such as the hypothalamus and thalamus.71,72 This reflex then has a parasympathetic output, resulting in the autonomic features of headaches. Hypotheses of how nVNS modulates this pathway include activation of the nucleus of the solitary tract and modulation via the hypothalamus, creating an inhibitory effect on the parasympathetic functions with relief of symptoms in trigeminal headaches and migraine.71,72
An initial open-label, single-arm study of 30 patients with migraine treated with an nVNS device as an abortive treatment, consisting of two 90-second doses at 15-minute intervals, demonstrated a 21% pain-free rate at 2 hours.65 Following this study, a randomized, double-blinded trial was completed. The PRESTO trial included 243 patients with episodic migraine with and without aura and randomized them to receive nVNS or sham within 20 minutes from pain onset.73 The nVNS device was superior to sham to achieve pain freedom at 30, 60 and 120 minutes, leading to the approval of gammaCore® (electroCore, Inc., Rockaway, NJ, USA) by the FDA for the prevention and acute treatment of migraine headache and the acute treatment and adjunctive use as a preventive treatment for CH (Table 1).73,74
Recently, the results of the PREMIUM II trial were published, which aimed to evaluate the efficacy and safety of nVNS for migraine prevention.75 Even though the results for the primary endpoint of mean reduction in monthly migraine days did not reach statistical significance, secondary endpoints favoured nVNS. The percentage rate of patients with greater than 50% reduction in migraine days was greater in the treatment group.
The effects of nVNS stimulation in CH were reported in the ACT1 trial, a randomized, double-blind study that evaluated nVNS for acute treatment.76 The response rate of pain relief was analysed in patients with episodic and chronic CH. Patients with episodic CH treated with nVNS had a 34% response rate for pain relief at 15 minutes compared with 10.6% in the sham group. Following this, the ACT2 study showed similar results in a European setting.77 The response rate in patients with episodic CH treated with nVNS was 48% versus 6% in the control group, with patients in the treatment group achieving superior pain-free rates, responder rates and decreases in pain intensity.77
nVNS is also approved for adjunctive use in prevention treatment of CH.78 The PREVA trial demonstrated that patients treated with adjunctive prophylactic nVNS had a significantly greater reduction in number of attacks per week.78 nVNS is a technology with minimal side effects, which can include skin irritation, pain at the application site and nasopharyngitis.68
Transcranial magnetic stimulation
TMS was introduced in 1985 and has been broadly used in the diagnosis, monitoring and treatment of various neurological and psychiatric ailments.79,80 It is a non-invasive electrical stimulation that generates a transient magnetic field, which then projects to the underlying brain cortex, either activating or suppressing different brain functions including motor, sensory and cognitive areas.81
TMS consists of a pulse of electric current that passes through a coil within a device placed on a patient’s head.80,82 The magnetic field then passes through the skull and induces mild electric currents in the brain, which then excite and depolarize neurons. Cortical spreading depression is an intense depolarization of neuronal membranes and has been shown to be the underlying mechanism of migraine aura.80 Studies in animal models have demonstrated that TMS inhibits cortical spreading depression, therefore having the potential to terminate aura and reduce the duration and severity of migraine pain.80
There are two types of TMS depending on the method of stimulation: repetitive or single pulse stimulation. Repetitive TMS (rTMS) delivers repeated pulses of the same intensity applied to a single brain area at a given frequency.79 It has been shown to modulate cortical activity, and has an inhibitory effect at low frequencies and enhances cortical excitability at high frequencies.83 Pilot studies have shown that high-frequency TMS delivered to the dorsolateral prefrontal cortex was able to relieve chronic migraine.83 A meta-analysis of five RCTs indicated that TMS, specifically high-frequency rTMS, was effective at preventing migraine.84 However, the dose and frequency of stimulation were different in the trials, which prompts the need for standardization of stimulation parameters in future RCTs.
In contrast, preclinical studies have shown that single-pulse TMS (sTMS) raises the threshold for cortical spreading depression and modulates the activity of nociceptive thalamic trigeminal neurons.85 A randomized, sham-controlled trial showed that patients who used sTMS had increased pain relief at 2 hours, and the effect lasted for 24 and 48 hours after treatment, compared with a sham device.86 By 2013, the sTMS mini™ device (eNeura Inc., Baltimore, MD, USA) was FDA approved for acute treatment of migraine with aura.87
The multicentre, prospective, single-arm, open-label ESPOUSE study investigated sTMS for the prevention of migraine with or without aura.85 Forty-six per cent of the patients treated with the sTMS device had a greater than 50% reduction in the number of headache days. The study outcome prompted extension of the FDA approval for prophylactic treatment of migraine in adults, and subsequently for adolescents as well (Table 1).88,89
TMS is a non-invasive, painless intervention; the most common side effects reported are scalp discomfort and headaches.80 Overall, sTMS has been shown to have no harmful effect on brain tissue, blood pressure or cardiac rhythm. It is contraindicated in patients with epilepsy due to risk of inducing a breakthrough seizure; however, safety studies have reported no seizures secondary to its use in healthy individuals or in patients with a neurological disorder when applied to the occipital cortex.80
Conclusion
Various neuromodulation techniques have been evaluated for their effectiveness in the treatment of migraine and CH over the past few decades. The mechanisms, expected physiological effects and data surrounding the effectiveness of eight invasive and non-invasive neuromodulation techniques have been reviewed in this article. Neuromodulation has often been evaluated in patients who were refractory to traditional pharmacological treatment of migraine and CH. Positive studies have led to FDA approval of t-SNS, COT-NS, REN, VNS and sTMS for clinical use, and CVS has been granted de novo class II status in the USA. These devices can be used alone or in conjunction with existing pharmacotherapies. More research is needed to expand our understanding of the precise mechanisms through which neuromodulation techniques alter the pathophysiology of migraine and CH. Such knowledge would help refine the existing techniques and improve their efficacy and may allow for better personalization of treatment.