Although disease-modifying therapies have been the primary focus of multiple sclerosis (MS) treatment during the past two decades, there is increasing awareness of the importance of treating patients holistically, paying attention to functional comorbidities and addressing mood and cognition. In general, promoting healthy behaviours and encouraging physical activity is essential to enhancing quality of life (QoL) and a sense of wellbeing in patients living with MS. This review summarizes the up-to-date literature on the lifestyle factors that may impact MS disease burden, including diet and obesity, exercise, sleep and fatigue, vitamin D, smoking tobacco and stress.
Diet and obesity
While the precise relationship between diet and MS risk and severity is unclear, it is a topic that is frequently queried by patients with MS. Although many studies are limited by sample size or study design, it is becoming clear that dietary habits play a role in the severity, and potentially the incidence, of MS. Certain dietary factors may promote immune-regulation, potentially through producing effects on the gut microbiome,1 while others may be pro-inflammatory.2 An in-depth discussion of the effects of dietary factors on the gut microbiome is outside of the scope of this review, but more detailed information can be found elsewhere.3 Obesity and elevated body mass index (BMI) are well established risk factors for MS.4–6 A study by Brenton et al. demonstrated increased obesity in paediatric patients with early-onset MS compared with those with later disease onset.7 When compared with demographically matched healthy controls, children with MS had significantly higher BMIs beginning up to 11 years before MS onset and remaining significantly elevated every year up to diagnosis.8
A summary of notable dietary intervention studies in MS is presented in Table 1.9–12 Outcomes studied include relapse rates, metrics of mood, Expanded Disability Status Scale scores, fatigue metrics and QoL metrics. Much of the literature to date emphasizes low-fat diets, including Mediterranean and omega-3 polyunsaturated fatty acids (PUFA)-based diets, albeit with conflicting clinical results.
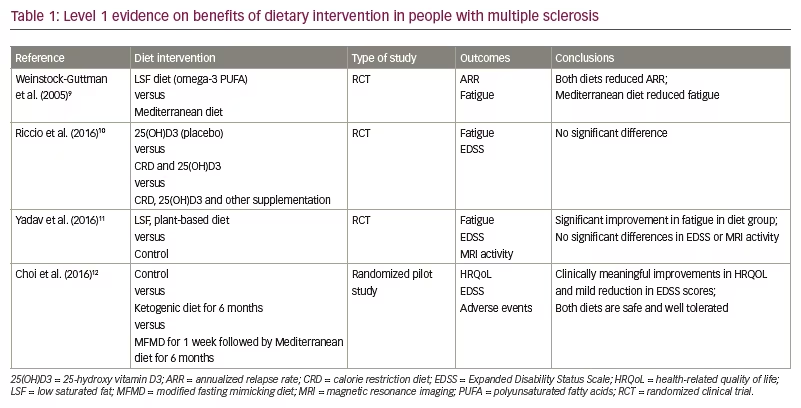
One notable multicentre prospective cohort study demonstrated that each 10% increase in energy intake from fat led to a 56% increase in the hazard of MS relapse, and each 10% increase in energy from saturated fats tripled this risk.13 In contrast, an increase of a one-cup equivalent of vegetables led to a 50% decrease in relapse risk.13 However, a diet low in saturated fat did not appear to improve Expanded Disability Status Scale score or magnetic resonance imaging (MRI) activity.11,14 It is unclear whether PUFA intake impacts MS severity, as clinical trials have produced conflicting results.14–17 High-fibre diets, such as those high in fruits and vegetables, have also been suggested to be beneficial in patients with MS, but studies are limited by a small sample size and lack of randomization.18–20
While diets such as the Wahls diet (a modified paleo diet)21 and the Swank diet (a very low-fat diet)22 have been popular amongst patients, the evidence for specific and highly restrictive diets such as these remains limited. A recent short-duration longitudinal study randomizing patients to either the Wahls or the Swank diet found that both diets reduced fatigue and improved QoL from baseline.23 However, this study was limited by the lack of a non-intervention control group, and it did not assess whether changes in fatigue/QoL were related to a decrease in BMI during the study. Given the differences between the two diets, weight loss could be postulated as a common contributor to the improvements in fatigue scores, as suggested by a prior placebo-controlled pilot studying the effects of a Mediterranean diet.24 However, given that both diets are high in fibre and focus on a high intake of fruits, vegetables and unsaturated fats, with a low intake of processed foods, it is likely that these common factors may be beneficial for MS-related fatigue.
Fasting-type diets have also gained popularity, although the evidence supporting their benefits is conflicting. Recent open-label studies have demonstrated improvements in several clinical disability metrics, but the lack of a control group heavily limited the interpretation of the results.25,26 A pilot study of 17 patients randomized to either intermittent fasting or a control diet showed no effects on clinical measures between the groups after 2 weeks.27 However, a longer 8-week randomized pilot study of 36 patients who underwent either daily calorie restriction, intermittent (2 days/week) calorie restriction or a control diet demonstrated substantial weight loss and improved emotional wellbeing in both CR intervention arms.28 Finally, a recent pilot study with a small sample size demonstrated improved serum neurofilament light chain levels after 6 months of an adapted ketogenic diet compared with controls, providing preclinical evidence of a possible neuroprotective effect.29 Further work is necessary to fully understand the relationship between these dietary modifications and MS and whether fasting-type diets offer an MS-specific benefit beyond weight loss alone.
While there is enough literature to recommend a diet for cardiovascular benefit – low in saturated fats, high in fibre and high in fatty fish – it is not clear which diet, if any, provides the highest benefits in MS due to limited evidence and conflicting study results, pointing to the need for further research to compare diets.13,21–23,25,27 However, there is general agreement in the literature that a diet high in fibre, vegetables, whole grains and fatty fish and low in saturated fat, sugar and processed foods can be potentially beneficial for patients with MS.
Exercise
Regular exercise is of the utmost importance in managing symptoms, restoring function and promoting wellness and QOL in people with MS.30 However, despite the growing body of evidence of these benefits, people with MS tend to exercise less than their healthy counterparts.30–33 This relative lack of exercise may reflect limitations related to ambulatory disability, pain, fatigue, environmental barriers or to depression and other psychosocial factors (e.g. self-efficacy, goal setting and social support). A recent evaluation of physical activity in young people with MS demonstrated that, while people with MS have positive perceptions of the benefits of exercise, this population also exhibited a fear that exercising could worsen chronic MS symptoms and/or lead to relapses. Other deterrents to exercise included lack of social support and time for exercise, and it was deemed that better education regarding specific exercises would be beneficial.34
Exercise has beneficial effects on cardiorespiratory fitness, walking mobility and balance.30 Additionally, moderate weight-bearing exercise has been shown to improve bone mineral density, which could help reduce fracture risk in the MS population.35 Flexibility-directed exercises are important to limit spasticity in the early stages of MS,36 and pelvic floor exercises can improve incontinence and sexual dysfunction.37
Outside of the physical benefits of exercise, there is conflicting evidence regarding its beneficial impact on fatigue, cognitive dysfunction and depressed mood, symptoms that are frequently exhibited by individuals with MS. A systematic review examining the effects of exercise on cognition did not find definitive evidence that physical activity was associated with improved cognitive function in people with MS, but this may be related to limitations and variability of clinical trials.38 Small pilot studies have suggested possible beneficial effects of exercise on cognition in people with MS.39,40 Two meta-analyses and one Cochrane review examined the effects of exercise on fatigue outcomes in people with MS, suggesting modest improvement in fatigue after exercise training, but showed overall heterogeneous results.41–43 Some commonstudy limitations included lack of adequate power, the variety of exercise interventions studied, selective reporting of outcomes and the absence of pre-screening for severe baseline fatigue. Of note, physical activity does appear to mitigate sleep dysfunction and fatigue in children with paediatric-onset MS.44 Finally, several meta-analyses have reported the consistent benefits of exercise on symptoms of depression in people with MS.45–47
Based on the above data, exercise is likely to have profound physiological benefits in people with MS and is potentially beneficial for cognition, fatigue, mood and QOL in general. Additionally, exercise may help prevent the development of obesity, which is known to be associated with higher disease severity.4–6 The National MS Society recently recommended that patients with MS undergo at least 150 minutes of exercise each week; however, progress towards that goal should be gradual to avoid worsening underlying symptoms, such as fatigue.48
Sleep and fatigue
Sleep disorders and fatigue are common in patients with MS and can precede the disease diagnosis in its prodromal phase.49 Common sleep disorders in MS include restless leg syndrome, periodic limb movements and sleep apnoea.50 Fatigue is an extremely common symptom associated with disease duration and a common cause of the inability to work in patients with MS.51 Insomnia is often associated with fatigue in MS, but one study found that the variance in fatigue could not be explained by insomnia, daytime sleepiness, depression or level of exercise, suggesting other factors at play.52 While the aetiology of MS-related sleep disorders is poorly understood, patients with disrupted sleep patterns display decreased functional connectivity in thalamic circuits.53 If sleep disorders are intractable in initial therapy, the patient should be referred to a sleep centre for formal evaluation.
Overall, sleep disorders adversely affect the QOL of people with MS by worsening fatigue and depressive symptoms.50,54–56 As the treatment of sleep dysfunction has been shown to improve MS fatigue,57 which is tied to QOL,50 the treatment of sleep disorders may improve overall QOL. Patients with MS and sleep disorders frequently report a decline in self-perceived cognitive function;58 however, one study failed to show differences in objective cognitive scores between those with associated sleep disorders and those without them.59
With regards to treating MS-related fatigue specifically, recent evidence suggests that pharmacologic interventions may not be superior to placebo.60 In fact, several studies have demonstrated that multimodal non-pharmacologic interventions, such as cognitive behavioural therapy and rehabilitation, can effectively improve fatigue, sleep disorders and depression in patients with MS.61,62
It is important to differentiate between primary fatigue (a direct result of MS) and secondary fatigue (caused by comorbid conditions) in MS. There are various potential drivers of secondary fatigue in MS, such as sleep disorders, pain, spasticity, bladder disorders, mood disorders and medication effects. Therefore, potentially contributory comorbid conditions and medications should be ruled out before behavioural interventions and therapeutic trials can be considered.63 A careful analysis of the factors contributing to sleep disturbance and/or fatigue is of utmost importance before considering lifestyle or pharmaceutical interventions.
Vitamin D
There are several genetic and environmental factors that can contribute to the risk of developing MS: one of particular interest is vitamin D.64 Vitamin D is prohormone derived from sun exposure, diet and supplementation.65 It has anti-inflammatory effects on both the innate and adaptive immune system.66 Vitamin D deficiency not only influences the risk of developing MS67–69 but has also been associated with MS disease activity,70–75 progression74–76 and the conversion from a clinically isolated syndrome to clinically definite MS.77 Furthermore, vitamin D deficiency in mothers during early pregnancy has been linked to a two-fold increased risk of their offspring developing MS in the future.68
Several studies have evaluated the impact of vitamin D supplementation on MS development and disease course.67–78 A large observational study examining vitamin D intake in relation to the risk of developing MS found that women who supplemented with vitamin D had a 40% reduced risk of developing MS over a 20-year follow-up period compared with those who had no supplemental vitamin D intake.67 However, the two largest studies to date – the SOLAR (Supplementation of VigantOL® oil versus placebo as add-on in patients with relapsing remitting multiple sclerosis receiving Rebif® treatment [SOLAR]; ClinicalTrials.gov identifier: NCT01285401)75 and CHOLINE (A multicentre study of the efficacy and safety of supplementary treatment With cholecalciferol in patients with relapsing multiple sclerosis treated with subcutaneous interferon beta-1a 44 µg 3 times weekly [CHOLINE]; ClinicalTrials.gov identifier: NCT01198132)78 studies – evaluating the effects vitamin D supplementation as add-on therapy to interferon beta-1a failed to show an effect on clinical parameters such as relapse rate and disability worsening.
Despite the inconsistent results regarding supplementation, there remains evidence to support the role of vitamin D in reducing MS severity. Prospective and retrospective studies have shown a reduction in relapse risk in individuals with higher serum vitamin D levels, with the risk further declining with every 10 ng/mL (25 nmol/L) increase in serum level.70–72 Higher vitamin D levels have also been associated with a reduction in disease activity on brain MRI, showing a protective effect on the development of new T2 lesions and contrast-enhancing lesions.73–75
The current evidence suggests that vitamin D is a modifiable risk factor and highlights the importance of preventing vitamin D deficiency in MS. While the correction of vitamin D deficiency in people with MS is recommended, the optimal level of vitamin D is currently unknown. We recommend regular monitoring of vitamin D levels in patients with MS, with the avoidance of both deficiency and over-replacement.
Smoking tobacco
Several studies have demonstrated that smoking tobacco is associated with a higher risk of developing MS and a higher disease burden following a diagnosis. In 2001, the Nurses’ Health Study demonstrated not only that smokers exhibited a higher risk of developing MS than non-smokers but that the number of cigarettes and duration of exposure were also predictive factors.79 Another large meta-analysis demonstrated similar findings, showing a higher odds ratio of developing MS related to increased cigarette exposure, a risk that appeared to persist even in ex-smokers.80 Interestingly, passive smoke exposure (i.e. second-hand smoke exposure) has also been reported as an independent risk factor for MS development.81 Finally, with an established MS diagnosis, smoking has been associated with faster disease progression and accumulation of disability compared with non-smoking,82 and smoking cessation has been associated with improved outcomes.83
The relationship between smoking and the development and severity of MS is incompletely understood. It is thought that it is likely resultant of pro-inflammatory and autoimmune effects and potentially arises from changes in the translational processing of immunological proteins.84 Furthermore, studies have demonstrated that certain components of cigarette smoke are toxic to oligodendrocytes and neurons,85 while nicotine itself may have protective properties.86 Given the definitive deleterious relationship between smoking tobacco and MS, special efforts should be made to counsel active smokers with MS and highly recommend cessation. Information on smoking cessation support resources can be found on the Centers for Disease Control and Prevention website.87
Stress
Stressful life events, conflicts and disruptions of routine have been associated with disease activity in MS.88,89 Hence, it is recommended that patients with MS take steps to limit stress. A randomized study that implemented a stress-management programme in patients with MS found that the programme significantly decreased perceived stress and depression compared with no stress-management intervention.90 In another randomized trial, stress management led to decreased rates of brain lesion formation.91 A systematic review of three randomized controlled trials found that mindfulness-based interventions led to improvements in QOL, mental health and fatigue.92 This suggests that stress-mitigation strategies could be a valuable tool in the treatment armamentarium for MS-related symptoms.
Conclusions
The treatment landscape in MS has been transformed in the past two decades with a myriad of effective pharmacologic therapies that have reduced the incidence of relapses and resultant disability. However, people with MS still often live with disabling symptoms. Despite the emphasis on pharmaceuticals, there is growing evidence that lifestyle measures are immensely important not only in mitigating the symptoms of MS but also perhaps in delaying or preventing disability.
A healthful diet low in saturated fats, processed foods and sugar, and high in fibre, vegetables and fatty fish is recommended; however, further research is necessary to determine whether certain specific diets provide more benefit than others. Avoidance of obesity, regular cardiovascular exercise exceeding 150 minutes per week, adequate sleep, adequate vitamin D level and avoidance of tobacco smoke are all important lifestyle factors that clinicians should regularly address with patients. Additionally, stress reduction techniques, such as mindfulness, may also be beneficial in mitigating symptoms. Given the need for comprehensive care, a multidisciplinary approach to MS is recommended. Neurologists can receive help from dieticians, physical therapists and physiatrists, psychologists, and primary care physicians to fully address lifestyle measures. Future research will serve to better elucidate the mechanisms by which these factors exert their effects on MS.