Prevalence
The prevalence of narcolepsy with cataplexy occurs with high variability, depending on the study population, and is thought to affect approximately 0.02–0.18% of the general population in Western nations.1,5 The true prevalence of narcolepsy without cataplexy is unknown and has been reported to range from 1%6 to 36%7 of narcolepsy cases, but may be as high as 50% of all narcolepsy cases.1 Diagnostic criteria for narcolepsy with or without cataplexy are a mean sleep latency (eight minutes and (two SOREMPs on the Multiple Sleep Latency Test (MSLT),8 according to the International Classification of Sleep Disorders.1 These criteria, however, have been observed in the general population of the Wisconsin Sleep Cohort Study9 in 5.9% of men and 1.1% of women.10 Singh and colleagues have shown that in a random sample of 333 adults who had an MSLT of (five minutes, 9.5% had two or more SOREMPs.11 These data, along with others reporting the presence of SOREMPs in non-narcolepsy patients,12,13 have led some authors to suggest that SOREMPs may be more closely related to ES than narcolepsy, and that re-evaluation of the International Classification of Sleep Disorders diagnostic criteria may be warranted.10 The age at onset of narcolepsy follows a bimodal relationship, with a first major peak at 15 years and a second smaller peak in the mid 30s.14 ES typically appears as the first symptom, with the first occurrence of cataplexy delayed by five years.14
Pathophysiology
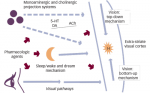
In patients with cataplectic narcolepsy, 78–100% exhibit abnormally low circulating levels of the hypothalamic neuropeptide hypocretin-1,15-18 perhaps due to an autoimmune process, since a strong concordance between low hypocretin-1 levels and the DQB1*0602 subtype has also been shown.16-20 Reports of hypocretin-1 levels in patients with noncataplectic narcolepsy have shown variable results across studies,16-18 and the HLA DQB1*0602 allele is present in only 41% of patients without cataplexy.21 Diagnosis
Narcolepsy with or without narcolepsy is primarily diagnosed by a clinical history of excessive daytime sleepiness that occurs daily for three months.1 Polysomnography (PSG) is performed to rule out other sleep disorders such as obstructive sleep apnea. The nocturnal PSG is followed by an MSLT, in which a mean sleep latency of (eight minutes and SOREMPs on (two occasions is required for diagnosis.1 Diagnosis of narcolepsy with cataplexy also requires a history of cataplexy. Cerebrospinal fluid hypocretin-1 levels less than one-third of mean normal values may be used rather than the MSLT/SOREMP criteria in patients with cataplexy.1
Consequences of Narcolepsy
Patients with narcolepsy have considerably diminished quality of life,22,23 and the majority of these effects are directly related to ES23. Impaired memory 1,24 and impaired performance related to vigilance and cognition 24-27 are common complaints that affect more than half of all patients with narcolepsy.24 Patients with narcolepsy are at substantial risk for automobile accidents, with nearly one-third of patients reporting accidents compared with 5% of non-narcolepsy controls.24 The ES and unwanted sleep episodes associated with narcolepsy can result in substantial impairments with work and life activities.22-24,28 In one study, nearly half of patients reported decreased earnings and fear of job loss, and more than one-third reported lost promotions as a result of their disorder.24 Unemployment because of narcolepsy was reported to occur in 20–43% of patients with narcolepsy.22,24,28 Narcolepsy also compromises social and family relationships, and almost 20% of patients report being bullied or teased, having difficulty making friends, and losing relationships (including divorce) because of their disorder.22,24
Current Treatment Options
Improved sleep hygiene and scheduled naps throughout the daytime hours are examples of lifestyle changes that are used to manage narcolepsy. In addition, management may include the prescription of drugs for treating ES and cataplexy (see Table 1). The earliest available treatment options for narcolepsy included stimulants for ES and antidepressants for cataplexy. Within the past decade, modafinil was introduced for the treatment of ES, and sodium oxybate was subsequently approved for the treatment of cataplexy and later for ES.Armodafinil is an emerging drug for ES associated with narcolepsy that has not yet been approved for use.
Modafinil
Modafinil is a wake-promoting agent that is structurally distinct from central nervous system stimulants.29-31 Several studies have shown that modafinil is effective and welltolerated for the treatment of ES associated with narcolepsy.32-35 In short-term (6–12 weeks), double-blind, placebo-controlled studies of patients with ES associated with narcolepsy (with or without cataplexy), modafinil (up to 400mg/d) improved both objective (Maintenance of Wakefulness Test (MWT) or MSLT) and subjective (Epworth Sleepiness Scale (ESS)) measures of wakefulness.32-34,36 The effects on wakefulness observed with modafinil treatment were maintained throughout two 40-week open-label extension studies.35 Some patients who experience late-day ES may require dosing of modafinil in the morning and afternoon.37,38 Modafinil has been shown to significantly improve overall clinical condition 33,34 and cognition32 compared with placebo. Modafinil has also been shown to improve health-related quality of life in patients with narcolepsy in long-term studies.35 The number of cataplectic attacks were unchanged with modafinil treatment.32,36,39
In clinical studies, modafinil did not adversely affect desired sleep,32-35,40 and the most common treatmentrelated adverse events associated with modafinil were headache, nausea, and nervousness in patients with ES associated with narcolepsy.35 Modafinil has not been associated with rebound hypersomnolence. Modafinil has a low potential for abuse and has been classified as a Schedule IV controlled substance.41-43Stimulants
Stimulants (e.g. amphetamines, methylphenidate, and methamphetamine) have been recommended for patients with ES associated with narcolepsy as monotherapy or augmentation therapy.44-46 Stimulants have been evaluated in clinical studies for the treatment of ES in narcolepsy since the 1950s. A thorough review is beyond the scope of this editorial and the reader is referred to the American Sleep Disorders Association (ASDA) Standards of Practice review published by Milter et al.46 Since the publication of the ASDA review in 1994, no additional published clinical studies of the efficacy of stimulants in narcolepsy were identified through a literature search.
Few controlled studies have evaluated the efficacy of stimulants in patients with narcolepsy with or without cataplexy.47-49 In a one-week placebo-controlled study evaluating the effect of methylphenidate, pemoline, and protriptyline in 26 patients with narcolepsy, methylphenidate 60mg was the only drug to significantly improve patients’ ability to stay awake as measured by the MWT.47 In a 28-day, double-blind, placebocontrolled, crossover study of eight patients with narcolepsy, methamphetamine (20–60mg) was shown to improve wakefulness and deficits in performance; however, the difference in these effects was not statistically different to eight matched controls.48 While the effects of stimulants on reducing the number of cataplectic attacks is inconclusive, some patients have reported improvement in cataplexy with stimulant therapy.49 The effect of stimulants on sleep architecture has not been reported to date in patients with narcolepsy. Common adverse events associated with this class of agents include headache, nervousness, and gastrointestinal complaints.44,46 High doses of stimulants can result in psychosis, tachyarrhythmias, and decreased appetite.50 Tolerance with amphetamine and methylphenidate have been reported, and patients may require higher doses for efficacy.44 Stimulants are classified as Schedule II controlled substances.
Sodium Oxybate
Sodium oxybate is the sodium salt of gammahydroxybutyrate (GHB), a central nervous system depressant (and endogenous neurotransmitter) used for the treatment of cataplexy and ES in patients with narcolepsy. Sodium oxybate is thought to be a metabolite of gamma-aminobutyric acid (GABA) and is proposed to exert its effects through both GHB and GABA receptors. It should be administered as part of a regimen that includes modafinil or a stimulant. In a four-week, double-blind, placebo-controlled study of 136 narcolepsy patients on stable doses of stimulants, sodium oxybate (3–9g/night in two equally divided doses) improved ES and reduced the number of cataplectic attacks and night-time awakenings.51 These effects were maintained with sodium oxybate 6g/night in an extended 12-month open-label study.52
Adverse events associated with sodium oxybate included nausea, vomiting, dizziness, and urinary incontinence;51 there was no evidence of rebound cataplexy upon discontinuation after long-term treatment.53 There is little evidence of withdrawal syndrome after prescribed usage of sodium oxybate;54 however, withdrawal symptoms following excessive use can be severe.55,56 Administration of sodium oxybate requires two doses, one at bedtime and another 2.5–4 hours later. Both doses should be taken while the patient is in bed.57 Sodium oxybate has been classified as a Schedule III controlled substance.
ES=excessive sleepiness;
NA=not applicable;
QoL=quality of life;
SSRIs=selective serotonin reuptake inhibitors.
*Approval for ES is pending.
†Status will be determined upon approval.
Tricyclic antidepressants have been investigated in small, open-label studies to treat cataplexy and sleep paralysis associated with narcolepsy, but the results are variable.58 In a case series of 75 patients evaluated over a 4–7 year period, clomipramine was rated as ‘excellent’ or ‘good’ in reducing cataplexy in 88% of patients, and response was maintained for up to seven years.59 Among 14 nonresponders to clomipramine, 10 subsequently had ‘excellent’ or ‘good’ decreases in cataplexy with clonazepam. In other studies, fluoxetine was considered equally effective in reducing cataplexy and sleep paralysis compared with clomipramine in five of 11 patients also taking stimulants.60 In contrast, fluvoxamine was less effective than clomipramine in 18 patients.61 Citalopram in conjunction with a stimulant was reported to reduce cataplexy in three patients with narcolepsy.62 None of these agents has been assessed for improving ES associated with narcolepsy. Commonly reported adverse effects associated with antidepressants included nausea, dry mouth, indigestion, and increased weight.59-61 Rebound cataplexy was mentioned by the investigators as a consequence of the discontinuation of clomipramine, fluvoxamine, and clonazepam.59,61 Antidepressants are not classified as controlled substances.
Armodafinil
Armodafinil is the R-enantiomer of racemic modafinil, which consists of equal amounts of R- and S-enantiomers. Pharmacokinetic studies have shown that R-modafinil has a significantly longer plasma half-life than the S-enantiomer (10–14 hours versus 3–4 hours, respectively).63,64 In a 12-week, multicenter, double-blind, placebo-controlled study of 196 patients with ES associated with narcolepsy (with or without cataplexy), armodafinil (150 or 250mg/day) improved wakefulness at final visit for both early-day (0900–1500 hours) and late-day (1500–1900 hours) time points.65 Armodafinil also showed significant improvement in overall clinical condition and cognition (e.g. memory and attention) and reduced fatigue compared with placebo at final visit. Armodafinil had no effect on the number of cataplectic attacks nor was there any adverse affect on sleep integrity or sleep architecture. Headache, nausea, and dizziness were reported as the most common adverse events. Potential rebound effects upon withdrawal of armodafinil were not reported.
Conclusions
Research of narcolepsy with and without cataplexy has enhanced our understanding of the physiological basis for this disorder and the symptoms associated with it. Studies have revealed a strong correlation between low levels of the neuropeptide hypocretin-1 and symptoms of narcolepsy, as well as a strong correlation between the HLA DQB1*0602 genotype and cataplexy in patients with narcolepsy. Further research regarding the pathophysiology of narcolepsy is needed. Sleep hygiene and frequent daytime naps are useful non-pharmacological treatments for patients with narcolepsy.
Pharmacological treatment options for ES associated with narcolepsy have expanded from early treatment options of stimulants and antidepressants to include modafinil and sodium oxybate. Modafinil or stimulants are recommended as initial therapy to treat ES. Sodium oxybate may be beneficial in patients who require treatment augmentation for symptoms of cataplexy and/or ES; antidepressants have also been beneficial for the treatment of cataplexy. Armodafinil represents a potential new therapeutic option that maintains wakefulness throughout the waking period without adversely affecting intended sleep. The risks and benefits associated with each of these agents need to be carefully evaluated for individual patients. Continued enhancement of the understanding of narcolepsy through clinical and basic research will enable enlightened treatment approaches and improved clinical success.