Seizures are one of the most frequent neurological disorders in neonates − the incidence of seizures in infants born at term is 1–3 per 1,000 live births, and is even higher in both preterm and very-low-birth-weight infants at 1–13 per 1,000 live births.1 Seizures may signify serious malfunction of, or damage to, the immature brain and constitute a neurological emergency demanding urgent management.
Although there is no consensus on the ideal treatment of neonatal seizures, phenobarbital (PHB) remains the most popular first-line treatment.2,3 However, PHB and phenytoin often fail to control seizures with the first dose.4,5
Moreover, PHB is associated with several harmful side effects, both immediate – such as sedation, hypotension and respiratory depression – and long term, with concerns about its potential to adversely affect psychomotor development and neurological outcomes in the developing brain.6,7 For these reasons, the search for an alternative first-line therapy for neonatal seizures began in the last two decades.
Levetiracetam (LEV) is a newer anti-seizure medication (ASM) approved for the treatment of focal seizures in Europe since 2000 in infants older than 28 days and is increasingly being used to treat neonatal seizures.8 LEV appears to have excellent tolerability in neonates.9 It has good efficacy and an excellent safety profile.10,11 Unlike PHB, it does not cause sedation and has no recorded adverse cardiovascular effects. Although uncommon, a recognized side effect of LEV is increased irritability and tiredness. In this systematic review, we aimed to compare the efficacy and safety of both medications in the treatment of neonatal seizures.
Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.12 An agreed protocol was established and followed. The details of the PRISMA checklist are provided in the supplemental table (Table S1).
Eligibility criteria
We included all relevant studies, including observational and randomized controlled trials, that reported on the outcomes of interest for this review. The included articles compared the use of PHB versus LEV in the treatment of neonatal seizures. Studies that reported the use of a single agent, either PHB or LEV, were excluded from our analysis. There were no limitations regarding the publication date or language.
Search strategy
We conducted an extensive search of the online published literature in January 2024 for potentially relevant studies. We used the following search terminologies in various combinations to produce our search results ([‘neonates’ OR ‘new-born’ OR ‘new born’ OR ‘full term’ OR ‘full-term’] AND [‘seizures’ OR ‘convulsions’ OR ‘fits’] AND [‘phenobarbital’] AND [‘levetiracetam’]). We searched the following online databases: PubMed, Ovid MEDLINE, SCOPUS, EMBASE, CINAHL, Google Scholar and the Cochrane Library. We also searched the grey literature by searching the bibliographies of included studies for potential citations. There were no restrictions applied to the search in terms of date, language or publication types. We limited our search to studies performed on humans only.
The eligibility of studies produced from the initial search to be considered for this review was assessed by the first author (Hiba Bashar) and the second author (Khalid Bashar), according to the pre-agreed protocol. The abstracts from the initial search were read in full before a decision was made on whether to exclude an article. In the cases where the first and second authors disagreed about a certain study, the article was examined in full. Any remaining disputes were settled following a consultation with the senior author (Alison Walker). After excluding irrelevant studies, the full articles of the remaining studies were obtained and analysed for outcomes of interest to this review.
The main objective of our review was to compare the efficacy of both drugs in controlling neonatal seizures during the acute phase. We also compared PHB and LEV in terms of safety by assessing the rate of complications and adverse reactions. Our secondary objective focused on the effects of PHB and LEV on long-term development, including motor and language development, as well as cognitive function.
Data collection
The first (Hiba Bashar) and second (Khalid Bashar) authors independently assessed data extracted from individual studies. All relevant data were extracted on a Microsoft Excel spreadsheet. The first two authors met regularly to discuss progress and settle disagreements about the included data. The senior author was then consulted to resolve any possible disputes or ambiguities related to the collected data and to settle any possible differences between the first two authors.
Demographic data were also recorded, including gestational age, gender, types of seizures, aetiology of seizures wherever possible, drug dose and the duration of follow-up. In addition, whenever possible, data were pooled to perform meta-analysis.
Quality assessment for risk of bias
The assessment of the quality of individual studies was performed by the first author (Hiba Bashar). As most of the studies included in this review were retrospective observational studies, we decided to use the Downs and Black tool to assess individual articles.13 This tool has widely been validated in the literature and consists of 27 different questions assessing the internal and external validity of included studies, along with bias and confounding factors. The original tool uses five questions to assess the inclusion of sample size calculations. For simplicity, we chose to combine those questions regarding sample size calculations into one. Therefore, our modified tool will result in a maximum score of 27 points, with lower scores indicating poor quality studies. The results of the quality assessment are provided in a supplemental table (Table S2).
Data analysis
Data from seven of the individual studies were pooled into a meta-analysis using Review Manager version 5.2.14 We opted to use the random-effects model of DerSimonian and Laird to compare categorical data, which were pooled as odds ratios.15 The Cochran’s Q-test was used to assess statistical heterogeneity.16 A cut-off of p<0.05 was used to report statistically significant differences.
Results
Study selection
The PRISMA flow diagram summarizes the process used in the literature search and the selection of studies for inclusion in this review (Figure 1). First, the search criteria detailed earlier were used to create the primary list of potential studies. This generated 181 articles. Restricting the search to studies performed on humans reduced the number to 138 articles. We did not restrict our search by language; however, all the included articles were published in English. In addition, there were no restrictions in terms of publication date.
Figure 1: Preferred Reporting Items for Systematic Review and Meta-analysis flow
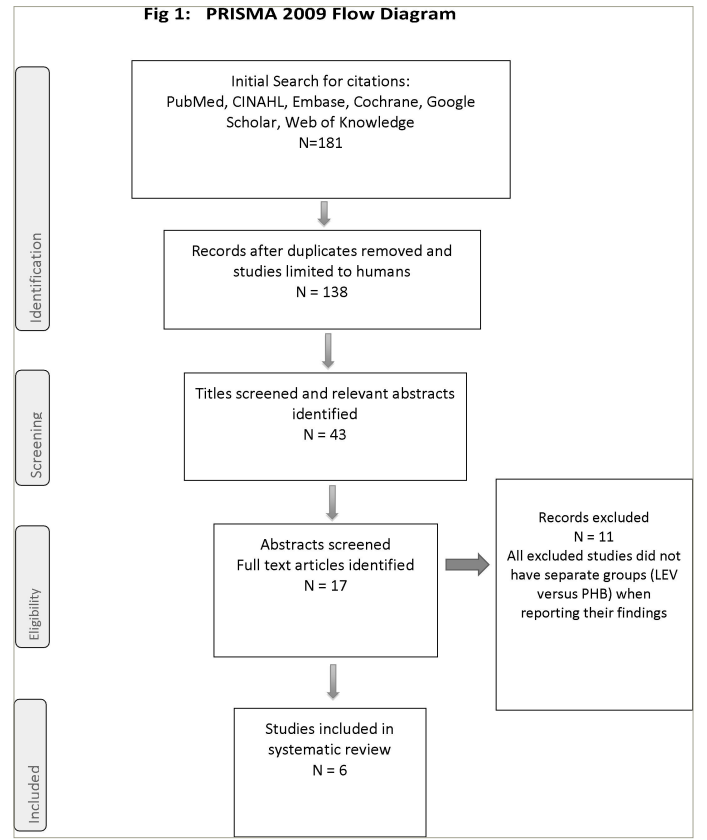
LEV = levetiracetam; PHB = phenobarbital.
We screened the titles and abstracts of all 138 eligible articles and reduced the number of potential studies to 17. After reading the full articles, we found that only six satisfied our agreed protocol.17–22 These six articles were then analysed in detail, and the findings are discussed below.
Participants
The total number of patients included in all studies was 420; of these, 200 were primarily treated with PHB, while 220 were treated with LEV as the first-line treatment. There were 186 (44.2%) female patients and 234 (55.8%) male patients. The characteristics of individual studies are summarized in Table 1.17–23
Table 1: Characteristics of studies17–23
| Studies | Design | Inclusion criteria | Exclusion criteria | Overview of cases and controls | Number and characteristics of cases (LEV) | Number and characteristics of controls (PHB) | Comment |
| Rao et al. (2018)17 | A retrospective cohort study | 44 patients who had VEEG-confirmed seizures; inclusion criteria were (1) greater than 36 weeks of gestational age, (2) less than 6 h of age, and (3) underwent therapeutic hypothermia with continuous VEEG monitoring | Clinical seizures without electrographic correlation | 78 newborn infants with HIE; 44 patients exhibited VEEG-confirmed seizures, of whom 34 became seizure-free after treatment; the remaining 10 patients died | Number of cases: 20. Gestational age: 39.0 (37.5–40.4) weeks. Birth weight: 3,123 (2,837–3,461) g. M/F=14/6 patients. APGAR score: 1 min; 1.0 (0.0–1.0) min. APGAR score: 5 min; 3.0 (2.5–4.0) min. APGAR score: 10 min; 5.0 (3.0–6.0) min | Number of cases: 24. Gestational age: 38.7 (38.0–39.7) weeks. Birth weight: 3,376 (3,081–3,565) g. M/F=15/9 patients. APGAR score, 1 min; 1.0 (0.0–2.0). APGAR score, 5 min; 3.0 (1.5–4.0). APGAR score, 10 min; 5.0 (2.0–6.0). The PHB group exhibited higher injury severity, with both higher ad hoc severity score (p=0.047) and higher proportion with short-term mortality (p=0.01) | Patients received PHB only (n=13), LEV only (n=18), LEV followed by PHB within 24 h of first LEV administration (n=2), or PHB followed by LEV within 24 h (n=10) after VEEG confirmation of seizures. The type of cooling method was not associated with demographic characteristics, seizure burden, injury severity, or the use of LEV versus PHB (all p>0.05). Accordingly, the selective head cooling and whole-body cooling subgroups were combined in all analyses |
| Falsaperla et al. (2019)18 | A randomized, one-blind prospective study | 30 neonates were included. Inclusion criteria were (1) term neonates and (2) seizures manifesting within the first 28 days of life. Seizures confirmed by EEG | 1. Requiring therapeutic hypothermia. 2. Seizures secondary to transient metabolic disorders. 3. Neonates with a positive history of maternal drug ingestion. 4. Those who received more than one anti-convulsant medication. 5. Those neonates in whom LEV was used as second-line therapy were excluded | 30 term neonates with seizures admitted to the neonatal intensive care unit of S. Bambino Hospital, University Hospital ‘Policlinico Vittorio Emanuele’, Catania, Italy, from February 2016 to February 2018 were randomized to receive PHB or LEV | Number of cases: 15. Gestational age: 38.13 ± 1.24. Sex (F/M): 4/11. Prenatal anomalies 40%. APGAR score: 1 min; 7.66 ± 1.29. APGAR score: 5 min; 9.13 ± 1.12 | Number of cases: 15. Gestational age: 38.33 ± 1.04. Sex (F/M): 8/7. Prenatal anomalies 40%. APGAR score: 1 min; 8.66 ± 0.89. APGAR score: 5 min; 9.03 ± 0.84 | The underlying aetiologies for seizure onset included hypoxic-ischaemic encephalopathy not requiring therapeutical hypothermia, stroke and central nervous system infections |
| Arican et al. (2020)19 | A retrospective non-randomized study | Term infants who were treated using PHB or LEV monotherapy as the first-line treatment for neonatal seizures and followed up in a paediatric neurology outpatient clinic. Seizures confirmed by EEG | Infants were excluded from the study group if they had received a second anti-epileptic drug in the neonatal intensive care unit or during the outpatient follow-up | The study group consisted of 62 infants who received monotherapy with PHB (n=22) and LEV (n=40); the mean duration of monotherapy was 8 ± 6 months. The mean time of the follow-up period in the paediatric neurology outpatient clinic was 19 ± 7 months (range: 9–42 months) | Number of cases: 40. Age (months; means ± SD) 18 ± 7. Sex (F/M) 21/19 | Number of cases: 22. Age (months; means ± SD) 20 ± 8. Sex (F/M) 13/9 | Neurodevelopmental assessments were carried out using the BSID-III by one of the co-authors of this research Mete Atasever. Neurodevelopmental outcomes were assessed by motor, cognitive and language performance in the BSID-III |
| Liu et al. (2020)20 | A retrospective cohort study | Inclusion criteria: (1) gestational age ≥35 weeks and birth weight >1,800 g; (2) occurrence of the first seizure in the first 28 days of life (in term infants) or by 44 weeks corrected gestational age (in preterm infants); (3) written consent from the parent(s)/guardian(s). Seizures were confirmed by EEG | Exclusion criteria: 1. PHB or LEV treatment duration of less than 3 days. 2. Seizures due to hypoglycaemia, hypocalcaemia, hypomagnesaemia or other electrolyte disorders | 125 newborns hospitalized for neonatal seizures between October 2014 and October 2018 at the Children’s Hospital of Chongqing Medical University | Number of cases: 59. Gestational age (weeks), mean (SD): 39.1 (1.1). There were no significant differences in the age of onset, seizure frequency, sex, gestational age, parturition or body weight between the PHB and LEV groups before admission (p>0.05 for all) | Number of cases: 66. Gestational age (weeks), mean (SD): 39.0 (1.0) | There were 28 patients who completed short-term treatment and 38 patients who completed long-term treatment in the PHB group; there were 12 patients who completed short-term treatment and 47 patients who completed long-term treatment in the LEV group |
| Sharpe et al. (2020)21 | A multicentre, randomized, blinded, controlled study | Patients were term infants of a corrected gestational age between 36 and 44 weeks (2 weeks of age) with a weight of at least 2.2 kg. VEEG-confirmed seizures | Patients were excluded if they had received any previous anti-convulsants (with the exception of short-acting benzodiazepines administered for sedation 24 h before enrolment), if the serum creatinine level was 1.6 mg/dL, or if seizures were due to correctable metabolic abnormalities. Patients in whom death was imminent were excluded; patients in whom EEG monitoring could not be commenced before the need to treat definite clinical seizures were not recruited | Randomly assigned (n=106) | Number of cases: 64. Sex (F/M): 31/33. Gestational age: 39.3 (1.3). 5 min APGAR score, mean (SD): 6.52 (3.01). Cord pH, mean (SD): 7.07 (0.2) | Number of cases: 42. Sex (F/M): 24/18. Gestational age: 39.1 (1.3). 5 min APGAR score, mean (SD): 6.47 (2.4). Cord pH, mean (SD): 7.15 (0.17) | NEOLEV2 (ClinicalTrials.gov identifier: NCT01720667) was an investigator-initiated, FDA-funded study23 |
| Thibault et al. (2020)22 | A retrospective single-centre study | Neonates (≤30 days of age, corrected gestational age ≤44 weeks) with electrographically confirmed seizures following cardiac surgery from 15 June 2012 to 31 December 2018; following the implementation of routine 48 h cEEG in all neonates following cardiac surgery with CPB | Authors did not provide exclusion criteria | Neonates with electrographically confirmed seizures managed with anti-seizure medication after cardiac surgery from 15 June 2012 to 31 December 2018 | Number of cases: 22. Sex (F/M): 12/10. Gestational age: 38 (37–39) weeks. Premature, n (%): 8 (37). Age at surgery, 4 (2–6) days. CPB time, 68 (45–89) min. Status epilepticus, n (%): 3 (14) | Number of cases: 31. Sex (F/M): 11/20. Gestational age: 38 (37–39) weeks. Premature, n (%): 11 (35). Age at surgery, 5 (3–8) days. CPB time: 46 (39–62) min. Status epilepticus, n (%): 10 (32) | Because of their anti-seizure properties, data were also collected regarding the concomitant administration of midazolam and ketamine for sedation or analgesia; no difference in concomitant midazolam; more neonates in LEV group received concomitant ketamine |
APGAR = Appearance, Pulse, Grimace, Activity and Respiration; BSID = Bayley Scales of Infant Development; cEEG = continuous electroencephalographic; CPB = cardiopulmonary bypass; EEG = electroencephalogram; F = female; FDA = US Food and Drug Administration; g = grams; h = hours; HIE = hypoxic-ischaemic encephalopathy; LEV = levetiracetam; M = male; min = minutes; PHB = phenobarbital; SD = standard deviation; VEEG = video-electroencephalogram.
Cessation of seizures
Rao et al. assessed 44 patients with confirmed seizures on video-electroencephalogram (VEEG).17 These patients were full-term neonates with mild-to-severe hypoxic-ischaemic encephalopathy (HIE) who underwent therapeutic hypothermia. The type of cooling used, whether whole body or selective head cooling, was not associated with any difference in terms of treatment effects. They found that the use of LEV was associated with a shorter duration to cessation of seizures compared with PHB (hazard ratio [HR]=2.58, p=0.007). This remained the case even after excluding patients who crossed over during the study. The severity of HIE was found to be an independent predictor of prolonged treatment duration (HR=0.16, p=0.001).
Liu et al. analysed 125 neonates retrospectively (PHB=66 and LEV=59).20 They set two time points for their analysis: 3 days (defined as short term) and 16 weeks (defined as long term). They found no significant difference between the two drugs in the short-term analysis (p>0.05). However, the neurodevelopment of those treated with LEV, as assessed by Gesell scores, was significantly better than those treated with PHB (p=0.026).
Sharpe et al. performed a multicentre, randomized, double-blinded study to assess both the safety and efficacy of LEV against PHB as a first-line treatment for neonatal seizures in term infants.21 All patients were randomly assigned and continuously monitored by VEEG. They reported that patients treated with PHB had a better rate of freedom from seizures within 24 h (p=0.001). However, increasing the LEV dose from 40 to 60 mg/kg resulted in a 7.5% increased efficacy of LEV in controlling seizures at 24 h.
Thibault et al. looked retrospectively at full-term neonates undergoing corrective cardiac surgery within 30 days of life.22 Seizure activity was confirmed on electroencephalogram. In their cohort, 31 neonates received PHB, while 22 received LEV as first-line therapy. Both drugs had similar efficacy in controlling seizures (PHB=13/31 versus LEV=12/22; p=1.0). Furthermore, although LEV required less time (1.5 h) to control seizures compared with PHB (5.2 h), the difference was not statistically significant (p=0.35).
Two of the included studies reported data on the cessation of seizures within 24 h in a way that allowed for a meta-analysis calculation.21,22 The two studies had 136 patients, with 75 neonates receiving LEV as first-line medication compared with 61 neonates in the PHB group. In the LEV group, 27 out of 75 neonates responded to treatment compared with 42 of 61 in the PHB group. This difference was not statistically significant in the pooled analysis (pooled odds ratio=0.29; 95% confidence interval [CI]: 0.03–2.45; p=0.26; Figure 2). There was significant heterogeneity detected (Cochran’s Q=7.65; degree of freedom [df]=1; p=0.006; I2=87%).
Figure 2: Cessation of seizures
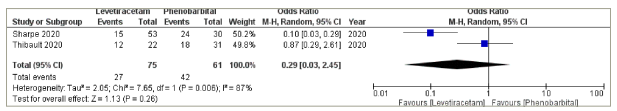
CI = confidence interval; M-H = Mantel–Haenszel.
Neurological assessment
Three of the included articles reported on outcomes related to neurodevelopment.18–20 Falsaperla et al. performed a randomized study (one-blinded) to investigate the use of PHB and LEV as first-line medications in the treatment of seizures in a group of term neonates within 28 days of birth.18 They used the Hammersmith Neonatal Neurological Examination (HNNE) in their assessment. They measured baseline scores at recruitment and compared them with scores measured 1 month following treatment. The overall scores improved significantly after 1 month of treatment when compared with scores at baseline in the LEV group (p=0.001). The same was true for individual scores used to measure tone and postures (p=0.05), reflexes (p=0.01), and orientation and behaviour (p=0.02). There was no significant improvement in the HNNE score in the PHB group in this study.18
Arican et al. studied term infants treated for seizures with either LEV (n=40) or PHB (n=22) as single agents.19 They treated their patients for a mean duration of 8 months and used the Bayley Scales of Infant Development (BSID-III) to assess their development. The difference between LEV and PHB groups was not statistically significant in any of the BSID-III score components (cognitive, motor and language).19
Liu et al. used the Gesell score at 16 weeks to investigate the effects of both LEV (n=47) and PHB (n=38) on neurodevelopment.20 The LEV group had better scores at the neurodevelopmental level (Gesell scores in response), with the difference being statistically significant (p=0.011). However, the difference was not statistically significant in language or motor function (fine motor and gross motor).20
Complications
Three of the studies reported adverse events and complications in patients receiving either LEV or PHB as a first-line treatment.20–22 Liu et al. found that after 16 weeks of treatment, neither of the two drugs was associated with abnormal blood tests, including renal function and/or liver function tests.20 However, in the LEV group, irritability (3/47, 6.38%) and feeding problems (3/47, 6.38) were the most commonly reported adverse events compared with somnolence (4/38, 10.53%) and feeding problems (2/38, 5.26%) in the PHB group.20
Sharpe et al. reported that adverse events such as respiratory suppression, hypotension, sedation and the need for inotropic support were more common in those assigned to receive PHB as first-line therapy.21 This remained the case even among those who received a lower dose of PHB (20 mg/kg). The differences were not statistically significant when compared with the LEV group; however, the study was not powered to make a definitive statement regarding adverse events.21
Thibault et al. reported that PHB was significantly associated with more adverse events when compared with LEV (p=0.006).22 Hypotension was the most common adverse event (7/43, 16.3%), whereas one patient suffered from severe respiratory depression and required non-invasive respiratory support. They found no association between adverse events and the peak plasma concentration of PHB, suggesting that those adverse events are not dependent on the treatment dose of PHB.22
A meta-analysis was performed using data from two of the individual studies to compare the risk of adverse events between the groups.17,22 They had a combined total of 97 participants, with 42 in the LEV group and 55 in the PHB group. One adverse event was reported in the LEV group in the studies compared with 10 in the PHB group; this difference is statistically significant (pooled odds ratio=0.15; 95% CI: 0.02–0.89; p=0.04; Figure 3). There was no significant heterogeneity detected (Cochran’s Q=0.68; df=1; p=0.41; I2=0%).
Figure 3: Adverse events
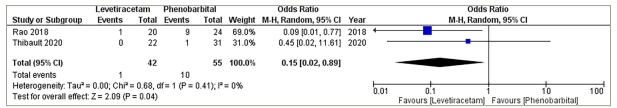
CI = confidence interval; M-H = Mantel–Haenszel.
Dosage
One of the main concerns with using LEV in the management of neonatal seizures is the calculation of the dose. Rao et al., in their study, used a daily median LEV dose of 21.3 mg/kg/day (20–30 mg/kg/day) initially, followed by a maintenance dose of 51 mg/kg/day (35–60 mg/kg/day).17 In the PHB group, they used a loading dose of 20 mg/kg/day (15–20 mg/kg/day).
Falsaperla et al. used an initial LEV dose of 20 mg/kg, followed by an oral maintenance dose of 20 mg/kg, which was gradually increased to 40 mg/kg twice daily in non-responders.18 A loading dose of 20 mg/kg was administered intravenously, followed by a maintenance dose of 5 mg/kg in the PHB group.
Similarly, Arican et al. used an initial LEV dose of 20 mg/kg, which was then increased by increments of 10 mg/kg up to 40–60 mg/kg for non-responders.19 In the PHB group, they used an initial intravenous dose of 20 mg/kg, which was increased by increments of 10 mg/kg up to a maximum dose of 40 mg/kg for non-responders.
Conversely, Liu et al. used a variable twice-daily dose of LEV (8–54 mg/kg, median=19 mg/kg) orally, while 5 mg/kg of PHB was given intravenously in repeated doses, twice or four times daily, for the first 3 d.20 A maintenance dose of 3–11 mg/kg (median=5 mg/kg) was then continued orally in split doses, two or four times a day, for the rest of the study period.
Sharpe et al. treated all their patients initially with an intravenous infusion of either LEV (40 mg/kg) or PHB (20 mg/kg) over 15 min.21 An additional infusion dose of the same medication was given over 15 min for those who did not respond to the initial dose: LEV (20 mg/kg) or PHB (20 mg/kg). Those in the LEV group then received a maintenance dose of 10 mg/kg intravenously every 8 h for 5 d, while those in the PHB group received a maintenance dose of 1.5 mg/kg intravenously every 8 h for 5 d. This method allowed the blinding to be maintained.
Thibault et al. reported that the initial LEV dose was similar among responders (30 [20–30] mg/kg) and non-responders (32.5 [20–50] mg/kg; p=0.54).22 Similarly, there was no significant difference between responders and non-responders treated with LEV as first-line therapy in terms of the number of doses (p=0.55) or the total loading dose (30 [20–50.5] mg/kg in responders versus 50 [40–60] mg/kg in non-responders, p=0.10). In the PHB group, they used an initial loading dose of 20 mg/kg (10–20 mg/kg) in non-responders, compared with 10 mg/kg (10–20 mg/kg) in responders (p=0.2). The non-responders in the PHB first-line group received more boluses (3–6, median=4) compared with responders (1–2, median=2), and this difference was statistically significant (p=0.00). In total, the non-responders received more PHB than responders (47.8 [30–50] mg/kg compared with 20 [19.2–25] mg/kg, p=0.005).
The main outcomes of the included studies are summarized in Table 2.17–22
Table 2: Main outcomes from studies17–22
| Studies | Seizure definition | Main reported outcome | PHB dose | LEV dose | Treatment duration | Complications |
| Rao et al. (2018)17 | VEEG-confirmed abnormal movement | Initial treatment with LEV, in comparison with PHB, predicted a shorter interval to seizure freedom in a univariate analysis (HR=2.58, p=0.007), even after adjustment for the initial seizure frequency and an unbiased ad-hoc measure of HIE severity (adjusted HR=2.57, p=0.010) | Median initial dose (‘load’) was 20.0 (15.0–20.0) mg/kg; median initial serum PHB level was 20 (18–33 μg/mL); and median maximum PHB level was 47.5 (31.0– 67.5) μg/mL | Median initial dosage was 21.3 (20.0–30.0) mg/kg/day; median maximum dosage was 51.0 (35.0– 60.0) mg/kg/day | Not specified | Not specified |
| Falsaperla et al. (2019)18 | Clonic or tonic–clonic seizures, ocular abnormal movements and subtle motor manifestations, such as tongue thrusting, cycling limb movements or apnoea; all included patients underwent serial VEEG recordings | The HNNE was used at baseline (T0) and again 1 month after the initial treatment (T1); the study showed a significantly positive HNNE score for the developmental outcomes, specifically tone and posture, in neonates treated with LEV (p=0.001); there was no significant improvement in the HNNE score at T1 in the neonates treated with PHB | IV PHB with an initial dose of 20 mg/kg, followed by a maintenance dose of oral PHB at 5 mg/kg | IV LEV at an initial dose of 20 mg/kg, followed by a maintenance dose of oral LEV at 20 mg/kg, with gradually increasing doses up to 40 mg/kg twice daily in case of non-response at initial doses | Therapy was maintained for 1 month after the seizures resolved | Not specified |
| Arican et al. (2020)19 | Abnormal movements confirmed by EEG | Both LEV and PHB therapy can be equally safe as monotherapy for neonatal seizures for the neurodevelopmental outcome assessment with BSID-III There was no statistically significant difference between PHB and LEV monotherapy subgroups | The initial dosage was intravenously 20 mg/kg, and the dosage was increased by 10 mg/kg up to 40 mg/kg in the case of persistent seizures | The initial dosage was intravenously 20 mg/kg, and the dosage was increased by 10 mg/kg up to 40−60 mg/kg in the case of persistent seizures | The mean duration of monotherapy treatment was 8 ± 6 months | There were no clinically relevant haematological, biochemical or vital sign parameter changes reported in any child; no patient discontinued anti-epileptic therapy because of treatment-related side effects |
| Liu et al. (2020)20 | Clinically diagnosed by an EEG | There was no significant difference between PHB and LEV treatment groups in short-term efficacy (p>0.05), but the cumulative survival function suggested that the LEV treatment group was better than PHB (p=0.026) in long-term efficacy evaluation; neurodevelopmental assessments at 16 weeks showed that LEV had a better effect on the neurodevelopmental level (Gesell scores in response) than PHB (p=0.011) | Repeated IV injection for 3 days at 5 mg/kg/day, given qd or bid, and then from 4 days to 16 weeks, was given orally at 3–11 mg/kg/day, in qd or bid (median dose, 5 mg/kg/day) | LEV was given orally at 8–54 mg/kg/day in bid (median dose, 19 mg/kg/day) | 16 weeks | Over the 16-week treatment period, the most frequently reported adverse events (>5% in the treatment groups) were somnolence (10.53%, 4/38) and anorexia (5.26%, 2/38) in the PHB group and irritability (6.38%, 3/47) and anorexia (6.38%, 3/47) in the LEV group |
| Sharpe et al. (2020)21 | Seizures were defined as an abrupt onset of rhythmic EEG activity lasting at least 10 s with a change in at least two of the following features: amplitude, frequency or spatial distribution | PHB was more effective than LEV in eradicating all seizures for 24 h (primary outcome measure); of the patients randomly assigned to PHB, 80% (24/30) remained seizure free for 24 h compared with 28% (15/53) of patients randomly assigned to LEV (p<0.001); 64% of patients randomly assigned to PHB remained seizure-free for 48 h compared with 17% of patients randomly assigned to LEV More adverse events occurred with PHB | Initial dose of 20 mg/kg, over 15 min, additional 20 mg/kg infusion over 15 min if seizures continued or recurred | Initial dose of 40 mg/kg over 15 min, additional 20 mg/kg infusion over 15 min if seizures continued or recurred | 5 days | Adverse events, including hypotension, respiratory suppression, sedation and requirement for pressor support, were more common in patients randomly assigned to PHB Patients who received only 20 mg/kg of PHB still experienced higher rates of adverse events |
| Thibault et al. (2020)22 | Seizures were defined as sudden and abnormal EEG events with a repetitive and evolving pattern with a minimum 2-μV peak-to-peak voltage and duration of at least 10 s | The cessation of electrographic seizures was similar in both groups, including 18 neonates (58%) with seizure cessation after PHB and 12 neonates (55%) with seizure cessation after LEV (p=1.0); the combined cessation rates of PHB and LEV when used as first- or second-line therapy were 58 and 47%, respectively (p=.47); PHB was associated with more adverse events (p=.006); eight neonates (14%) experienced an adverse event related to PHB use, including seven with hypotension and one with respiratory depression; no adverse events were reported with LEV use | Initial bolus: 20 (10–20) mg/kg; additional boluses 10 (5–10) mg/kg Total dose received in the first 72 h, 20 (19.2–25) mg/kg | Initial bolus: 30 (20–30) mg/kg; additional boluses: 22.5 (20–48.8) mg/kg Total dose received in the first 72 h, 30 (20–50.5) mg/kg | Not specified | Among the 43 neonates who received PHB, 7 neonates (16.3%) had hypotension attributed to its use that limited administration; one neonate was deeply sedated and had respiratory depression requiring the initiation of noninvasive respiratory support; there was no association between the presence of an adverse event and peak PHB plasma concentrations (p=0.67) |
bid = twice a day; BSID = Bayley Scales of Infant Development; EEG = electroencephalogram; HIE = hypoxic-ischaemic encephalopathy; HNNE = Hammersmith Neonatal Neurological Examination; HR = hazard ratio; IV = intravenous; LEV = levetiracetam; PHB = phenobarbital; qd = once a day; VEEG = video-electroencephalogram.
Discussion
To our knowledge, this is the first systematic review directly comparing the use of LEV versus PHB as first-line drugs in the treatment of neonatal seizures. Effectively managing neonatal seizures is paramount due to the significantly increased risk of adverse neurological sequelae later in life, including cerebral palsy, deafness, blindness and epilepsy.24,25 Traditional ASMs have included PHB, phenytoin and benzodiazepines; however, their efficacy and safety profile leave a lot to be desired.26–28 For instance, they have been shown to cause neuronal apoptosis in animal models and in vitro.29–31 Additionally, their association with neurodevelopmental outcomes when used in the treatment of neonatal seizures remains unknown.32 Hence, the search for alternative ASMs in the treatment of neonatal seizures, including more novel therapies such as LEV, continues despite the lack of comprehensive evidence to support the use of these new agents.27,33 This systematic review attempted to shed light on the evidence supporting the use of LEV in neonatal seizures from comparative studies.
PHB has long been considered the gold standard in the treatment of neonatal seizures; however, we believe that there is emerging evidence to suggest that LEV may not only be as effective but also safer.34 An earlier review by McHugh et al. found that LEV achieved a 77% rate of complete or near-complete cessation of seizures when used as the primary ASM compared with a 46% rate when PHB was used as the primary ASM.35
Our review demonstrates that LEV can be effective as first-line ASMs when compared with PHB. LEV was effective in reducing the number of seizures and was not found to be inferior to PHB with regard to complete cessation of seizures (p=0.26). We limited our review to articles reporting outcomes on full-term neonates, as preterm neonates have been shown to have higher rates of subclinical seizures as well as higher mortality.36 Moreover, there is evidence to suggest that seizures in preterm neonates can be aetiologically different.37
We have also shown that LEV is a safer drug when compared with PHB. One of the main concerns with traditional anti-convulsant therapies, particularly PHB, has been their deleterious effects on neurodevelopment in neonates later in life. Therefore, the search for ASMs with a safer profile, such as LEV, should be encouraged. Additionally, PHB was shown to be more associated with significant complications of hypotension and respiratory depression, in the studies included in our review.
Maitre et al. studied a cohort of 280 infants treated for neonatal seizures and reported that PHB was associated with worse BSID scores for cognitive and motor function.27 They reported the cumulative dosage from pharmacy records by calculating the sum of doses per body weight per day (mg/kg). The cognitive BSID score decreased by 8.1 points, and the motor BSID score decreased by 9 points per 100 mg/kg compared with 2.2 and 2.6 points per 300 mg/kg in the LEV group, respectively. The risk of developing cerebral palsy increased by a factor of 2.3 per 100 mg/kg in the PHB group (p=0.018), whereas LEV was not associated with an increased risk of cerebral palsy. Additionally, receiving any PHB was associated with lower BSID language scores (p=0.024), while a dose of LEV did not result in the same association.27
One of the main issues with using ASMs in neonates is calculating the appropriate dose. This was true for both LEV and PHB and was also true for calculating the initial dose and the maintenance dose, as well as deciding on the duration of treatment. Tulloch et al. examined the pharmacokinetics of ASMs used in the treatment of refractory seizures in a systematic review.38 The review assessed the use of eight different ASMs, including LEV and PHB, as second-line therapy. They concluded that there was a lack of evidence with regard to the pharmacokinetics of those medications to guide the calculation of ideal dosages in treating neonatal seizures. They recommended avoiding fixed doses due to the rapid development of neonates and their organ systems.
Therefore, ASMs should be monitored, and their doses should be adjusted according to clinical and physiological developmental changes. Additionally, monitoring the serum concentration levels of ASMs may guide the calculations of appropriate dosage, avoid potentially toxic levels of ASMs and reduce the risk of dose-related adverse effects. Additionally, the serum levels of LEV are not regularly monitored by clinicians, and there is considerable variation in drug testing and monitoring for LEV across different centres. The same practice is not always true regarding PHB, as more clinicians seem to monitor the drug levels for adjusting the dose and assessing its effectiveness.
There were significant variations in the dosages used across the studies included in this review. However, consensus would suggest that an initial dose of 40 mg/kg of LEV seems to be both reasonably efficient and safe when used as a first-line medication. More studies with larger numbers are needed to make definitive recommendations with regard to the dosage and duration of LEV. The same applies to PHB, as there remain significant variations with regard to both the dose and duration of treatment concerning its use in neonatal seizures. The authors of this systematic review recommend using serum creatinine levels to monitor the renal function in neonates receiving LEV to aid in adjusting the dose in the face of the associated risk of acute kidney injury. This can be achieved using the neonatal modified Kidney Disease: Improving Global Outcomes (KDIGO) criteria.39
Thibault et al. suggested that the treatment effects could have been undermined by the fact that the non-responders were clinically more unstable, had more refractory seizures and had a higher chance of being exposed to extracorporeal membrane oxygenation.22 Those confounding factors could be addressed in the future by performing a randomized controlled study.
Our systematic review has a few limitations that should be highlighted. The majority of the data analysed in our review come from retrospective studies with all of their known innate weaknesses. Retrospective studies are associated with a higher risk of certain types of bias, such as selection bias and loss to follow-up bias. Two studies excluded infants who needed a second-line ASM.18,19 It is therefore likely that in those two studies, seizures that were most difficult to treat were not enrolled. Moreover, one of these studies excluded infants receiving hypothermia, which could have introduced bias.18
The included studies also used variable scores to assess the neurodevelopmental functions of the included subjects, making it impossible to pool data for meta-analyses. The pooled analysis performed in our review is limited by both the number of studies included and the heterogeneous nature of some of those studies when pooled together. We have previously acknowledged that the dosages used for both PHB and LEV varied among the included studies, but the protocols were similar enough to be included in our systematic review.
Conclusion
Our review suggests that the use of LEV, as a first-line ASM, is as effective as the more traditional PHB, albeit with fewer long-term complications. The number and quality of included studies make it difficult to draw definitive conclusions; therefore, large randomized and blinded studies are needed. However, the available evidence is adequate to support the use of LEV as a first-line treatment for neonatal seizures.













