Headache is the second most common reason children seek neurological care in the USA.1 Migraine can affect children of all ages, and by the age 10, it affects more than 5% of children in the USA.1 Before puberty, it affects both boys and girls equally; however, post-puberty migraine is more prominent in females at a 3:1 ratio.1 Chronic migraine, a more disabling form of migraine, has a prevalence of 0.6% in children aged 5–12 years, and 0.8–1.8% in children aged 12–17 years.2 Importantly, children from socioeconomically disadvantaged backgrounds are four times more likely to experience chronic migraine and these children are also more likely to miss school and have higher levels of disability.2 Although common, and at times disabling, pediatric migraine is eminently treatable and a growing number of options are available for the acute and preventive treatment of these attacks.
Early life predictors of migraine
A number of predictive childhood factors have a strong correlation with migraine later in life.3 Many of these are thought to potentially represent early life manifestations of migraine, others are unique or rare subtypes of pediatric migraine.
Colic
Infantile colic typically presents from birth to 4 months of age, characterized with recurrent episodes of irritability, fussiness, or crying that can last at least 3 hours a day, occur on 3 days a week for at least 3 weeks.3 Children with migraine are more likely to have experienced infantile colic, and women with migraine are more likely to have a baby with colic.3 In addition, babies with colic are at a higher risk to develop migraine without aura as adolescents.3
Other episodic conditions
There are three episodic conditions that are very closely related to migraine. Benign paroxysmal torticollis affects older infants; it presents with periodic attacks of head tilt, nausea, vomiting, fussiness and at times ataxia in older toddlers. It has been shown to be associated with the CACNA1A gene, associated with one subtype of hemiplegic migraine.4 Benign paroxysmal vertigo affects children in their pre-school age; it presents with periodic attacks of vertigo that typically last for several minutes. Cyclic vomiting syndrome is a diagnosis most commonly seen in elementary school age children; it presents with periodic attacks of frequent vomiting that are occasionally accompanied by abdominal pain and anorexia. Also of note is the fact that the presence of nausea accompanied by headache in children is the strongest predictor of missing school.2
Diagnostic considerations
Red Flags
When considering the complaint of headache in children, it is essential to rule out secondary causes of headache. Red flags for migraine in children include systemic symptoms (fever, altered mental status, meningismus, immunocompromised state) and neurological symptoms (focal neurological deficits on exam, seizure at onset of headache). Additionally, it is important to screen for signs of raised intracranial pressure such as papilledema, diplopia, extra ocular movement abnormalities, and visual field changes. Headaches that occur when supine or with valsalva exercise or cough may sometimes indicate intracranial mass or Chiari malformation.5 Sudden onset, or thunderclap headaches can be a presenting sign of reversible cerebral vasoconstriction syndrome. Visual symptoms can be characteristic of migraine aura; however, children who experience colorful hallucinations may in fact have a rare epileptic condition such as Panayiotopoulos syndrome or idiopathic occipital epilepsy of childhood.6 Headaches that worsen with sitting up or standing consistently may be associated with low cerebrospinal fluid pressure. Side locked headaches may also be associated with pituitary mass with or without other symptoms of endocrine pathology.7
Neuroimaging in headache
Although an initial study implicated an occipital location of headache in children with an increased risk of an intracranial mass,8 more recent literature determined this was not the case.9 Occipital headache has been found to occur in 6–20% of all healthy children and neuroimaging for recurrent headache in children with a normal neurological exam has a 0–4.1% yield for actionable findings. As most of the children that had abnormal imaging also had abnormal findings on their neurological exam,9 it is no longer recommended to scan all children who present with an occipital headache location, rather, the neurological exam should be a major determining factor when considering neuroimaging for
occipital headache.9
Diagnostic criteria
The International Classification of Headache Disorders, Third Edition (ICHD-3) has specific diagnostic criteria for pediatric migraine (Box 1).10 These criteria are similar to that of adult migraine; however, pediatric migraine is unique in that the duration can be shorter (2–72 hours), and the location is more often bilateral (rather than unilateral in adults), and importantly, the associated symptoms such as nausea, photophobia and phonophobia that are required for a migraine in adults can be inferred from the actions of young children. The Pediatric Migraine Disability Assessment (PedMIDAS, Box 2) is a validated instrument that has been used for measuring headache related disability in children.11 It asks a total of six questions to assess the amount of school days, or partial school days, missed due to headache; the amount of activity that was limited due to headache; and the amount of activity that was carried out, but not to the best of the child’s ability due to headache.


Lifestyle modifications for migraine
When seeing a child with migraine it is essential to discuss and address potential lifestyle changes that may be contributing to headache frequency and how these can be managed and modified in order to prevent further progression of migraine.
Sleep
Although it can be very difficult to establish and maintain regularity and homeostasis of a daily routine in childhood and adolescence, unpredictability and changes in daily schedules can be related to an increase in migraine frequency.12 Therefore, the first step in migraine prevention is always counseling children and their families on proper lifestyle management. It is important to encourage adequate amounts of sleep for age as well as a consistent sleep schedule that does not vary weekday to weekend. Adolescents have a physiologic sleep phase delay that causes them to stay up later and sleep in later as well.13 New recommendations from the American Academy of Pediatrics state that high school should begin no earlier than 8:30 am;14 however, only 17.7% of schools in the USA actually abide by this time frame.
Hydration
The second aspect of lifestyle that children and adolescents are encouraged to focus on is hydration. In a random sample of children and adolescents aged 6–19 years, 55% of them were found to be mildly dehydrated based on their urine concentrations.15 Increasing water intake has been shown to be associated with improved headache severity in adults with headache.16 It is important to advise to have a water bottle at school with them at all times. Similarly, fasting can provoke migraine17 and so it is important to encourage children and adolescents with migraine to have portable snacks with protein on hand.
Physical activity
The incorporation of regular physical activity is essential when discussing lifestyle as well. In an adult study, aerobic exercise for 40 minutes three times a week was shown to improve the frequency and severity of migraine attacks equally as effective as some preventive medications.18
Acute migraine treatment
The proper use and timing of abortive medications has to be discussed with patients and their families at length as proper abortive treatment may decrease the chances of progression to chronic migraine.19 A number of classes of medications have been shown to be acutely beneficial for migraine.
Nonsteroidal anti-inflammatory drugs
Ibuprofen is a level A recommended abortive treatment of migraine in adults and children.20 In a randomized controlled trial, ibuprofen was found to be superior to placebo in children as young as 4 years old for the acute treatment of migraine at a dose of 7.5–10.0 mg/kg given every 6 hours as needed.21 Naproxen has a longer half-life and has been found to be safe and superior to placebo, especially when combined with sumatriptan.21 The recommended dose of naproxen is 5–10 mg/kg every 12 hours as needed.
Acetaminophen
In a double-blind crossover study, acetaminophen was found to have superior efficacy as compared with placebo for acute migraine in children ≥4 years old; however, in terms of effectiveness it was less effective and slower in onset than ibuprofen.22
Antiemetics
Dopamine receptor antagonists such as prochlorperazine are helpful particularly when there is prominent nausea.23 Co-administration of diphenhydramine can decrease the likelihood of movement side effects.24
Triptans
Four of the seven triptans have been studied for use in children of ages 12–17 years old. They are almotriptan 12.5 mg by mouth, zolmitriptan 2.5 mg or 5.0 mg nasal spray, rizatriptan 5.0 mg or 10.0 mg melted tablet and sumatriptan/naproxen 100.0/85.0 mg given by mouth.20 Only one triptan is approved by the US Food and Drug Administration (FDA) for use in children ≥6 years old, rizatriptan at 5.0–10.0 mg. It is generally recommended to prescribe a triptan if migraine attacks are not controlled, or are inadequately controlled, by nonsteroidal anti-inflammatory drugs (NSAIDs). They are avoided in children with a history of stroke or peripheral vascular disease. There should be a baseline blood pressure check prior to prescription to rule out any undiagnosed hypertension. Although many adolescents are on other serotonergic medications due to mood or psychiatric disorders, there is no indication to restrict the use of triptans in these individuals as the two drugs act on different classes of serotonin receptors. Triptans act on 5-HT1b and 5-HT1d while serotonin syndrome generally results from activation of 5-HT2a receptors.25
Dihydroergotamine
Dihydroergotamine is an ergot alkaloid that has been used in the treatment of acute and chronic migraine. The oral form has poor bioavailability, it is therefore more commonly used as a nasal spray or intravenous inpatient infusion. It has been well studied in the adult population;26 however, there are two retrospective chart reviews published, as well as a randomized, double-blind, placebo-controlled, crossover study in pediatrics.26–28 All were conducted in an inpatient pediatric hospital and all of them found that short term treatment with dihydroergotamine is an effective treatment for patients with prolonged migraine without aura. A study in 2011 found that dihydroergotamine is well tolerated, and that longer treatments produce better outcome. Nausea is the most common adverse reaction, and its control is associated with better outcome.27
Medication overuse
Excessive or inappropriate use of abortive medications can lead to
medication-overuse headache. ICHD-3 diagnostic criteria for medication-overuse headache includes headaches occurring >15 days a month in a patient with a pre-existing headache disorder, regular overuse for >3 months of one or more drugs taken for acute treatment of headache, and not accounted for by another diagnosis.10 It is important to address this medication overuse as it contributes to primary headache progression as well as decreased responsiveness to preventive medications in these patients.29
Preventive migraine treatment
Prevention treatments should be considered when children begin experiencing six or more headache days a month, four or more headache days with some impairment, or three or more headache days with severe impairment.30 It is also important to consider prevention when the PedMIDAS score is greater than 30, which portends poor quality of life. When discussing prevention, it is very important to set expectations with the patient and their family. Specifically, the goal for prevention is a 50% reduction in attack frequency, severity and duration, and an improved response to acute medications.
In adults, level A evidence exists for sodium valproate, topiramate, propranolol/metroprolol, candesartan, botulinum toxic injections, neuromodulation, and anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies. When considering prevention for children and adolescents, it is important to consider co-morbidities such as hypertension, diabetes, obesity, anxiety, depression, or seizures.
Non-medical therapies
It is recommended to start with options that have lowest potential for adverse events—including nutraceuticals such as vitamin B2, magnesium and melatonin, and cognitive behavioral therapy.30 Nocturnal urinary levels of melatonin are decreased in patients with chronic migraine, and a randomized controlled trial in adults has shown that melatonin is more effective for migraine prevention than placebo, and also more effective than amitriptyline.31 There are prospective controlled trials underway that are looking at the efficacy of melatonin as an abortive as well as preventive treatment in adolescents with migraine (ClinicalTrials.gov identifiers NCT03150797 and NCT03597529). Riboflavin has been shown to be effective in one randomized controlled trial.32 In 2003, a randomized, double-blind, placebo-controlled trial looked at oral magnesium prophylaxis of headaches in children aged 3–17 years. It did not show whether magnesium was superior to placebo; however, magnesium did significantly reduce the number of headache days in this population.33 In early 2019, a single-pulse transcranial magnetic stimulation device was approved for the acute and preventive treatment of migraine in adolescents >12 years old.34
Antiepileptics
Topiramate is the only preventive medication that is approved for adolescents >12 years old.35 The dosing recommendation is 2–4 mg/kg/day, with a maximum of 200 mg nightly. Depakote has also been studied in migraine prophylaxis. It has been found to have an efficacy similar to that of topiramate; however, it has a larger side effect profile.36 The side effects are outlined in Table 1.32,33,35–40,44–46
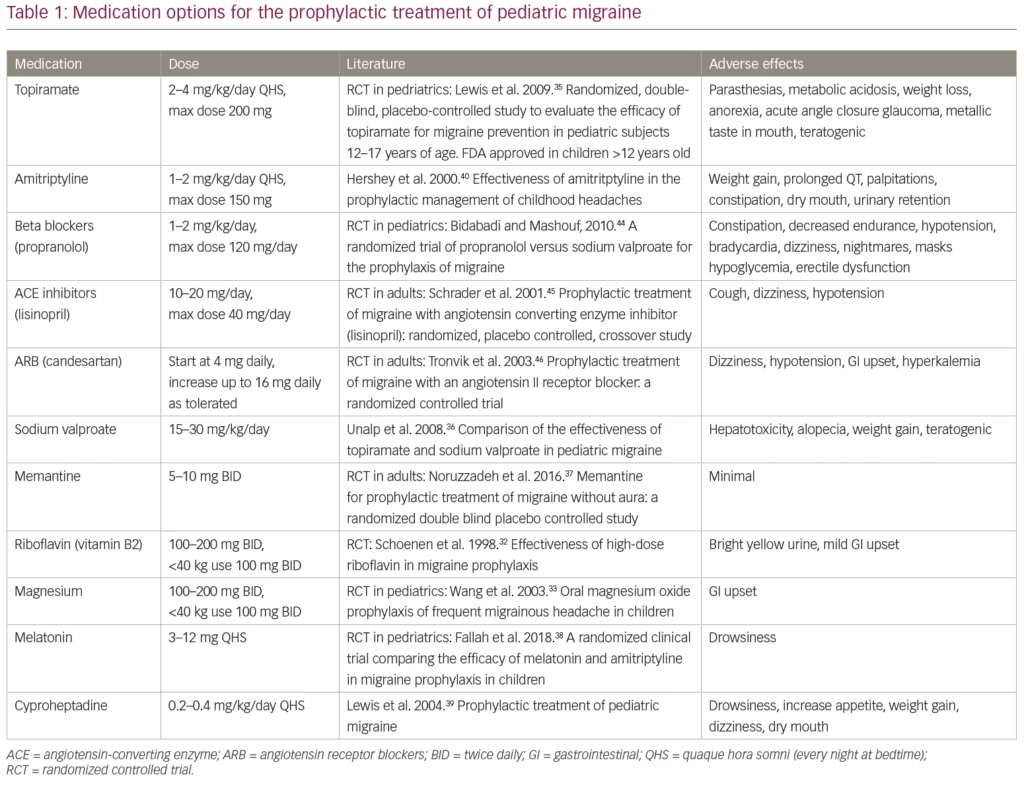
Antidepressants
Trials evaluating amitriptyline revealed a significant reduction in migraine days as compared with placebo.40,41 The dosing recommendation is
1–2 mg/kg/day to be taken at bedtime, with a maximum dose of 150 mg. Venlafaxine is a serotonin–norepinephrine reuptake inhibitor which has also been used for migraine prophylaxis in adults. An adult randomized, double-blind, crossover study of venlafaxine and amitriptyline found that venlafaxine was just as effective in migraine prophylaxis, but had a significantly more tolerable side effect profile.42
Blood pressure
Propranolol was found to be more effective than placebo, and just as effective as cyproheptadine in a three-way head-to-head migraine prophylaxis study.43,44 The recommended dose is 1–2 mg/kg/day. Lisinopril and candesartan are other medications in this class that have been commonly used for migraine prophylaxis in the pediatric population due to their low side effect profile; however, the data for their use is extrapolated from adult studies.45,46
Cyproheptadine
Cyproheptadine is commonly used in children <6 years old or weighing
<30 kg. The recommended dose is 0.2–0.4 mg/kg/day at bedtime. There is some evidence that it is effective in combination trials with other preventatives.43 Importantly, cyproheptadine has a category B safety level in pregnancy and is commonly used as a prophylactic agent in this population.
OnabotulinumtoxinA
OnabotulinumtoxinA is an FDA approved level A evidence method of migraine prevention in adults >18 years old. The PREEMPT 1 trial in adults showed that 50–70% of patients had a >50% reduction in migraine headache days per month.47 There are no randomized controlled trials that have been done in adolescents; however, a retrospective longitudinal analysis has shown significant improvement from baseline.48
Nerve blocks
Greater occipital nerve blocks with either bupivicaine or lidocaine have been shown, in multiple studies, to be effective in the treatment of chronic migraine.49,50 A randomized controlled trial in children with chronic migraine revealed that patients who received weekly greater occipital nerve blocks with bupivacaine had improvement superior to placebo for treatment of chronic migraine.51
Calcitonin gene-related peptide antagonists
Anti-CGRP monoclonal antibodies have recently been FDA approved for the treatment of migraine in adults. CGRP is primarily produced in nervous tissue, and its receptors are expressed throughout the body. Anti-CGRP monoclonal antibodies have been found to decrease the frequency of migraine by 50% a month in adult patients with high-frequency episodic migraine,52 and have a very low adverse side-effect profile. Randomized double-blind, placebo-controlled trials are currently underway in children and adolescents (e.g., ClinicalTrials.gov identifiers NCT03432286 and NCT03832998). Until this empiric data is available, members of the Pediatric and Adolescent Headache special interest group of the American Headache Society wrote a consensus for the recommendations of the use of anti-CGRP monoclonal antibodies in children.53 While randomized controlled trials are being conducted, the current recommendations are that these drugs are only considered for use in post-pubertal adolescents with relatively frequent migraine (more than eight a month), with a moderate-to-severe migraine disability as per PedMIDAS score. It is also recommended that patients have trialed at least 2–3 other prevention therapies, which include cognitive behavioral therapy and nutraceuticals.
Behavioral therapies
The Childhood and Adolescent Migraine Prevention (CHAMP) trial, a large, National Institutes of Health-sponsored, multicenter, randomized controlled trial, was completed in 2017 to evaluate preventive migraine therapy in adolescents aged 12–17 years.54 Inclusion criteria were episodic migraine or chronic migraine with a PedMIDAS score of ≥10. The participants were divided into three groups in a 2:2:1 ratio for topiramate:amitriptyline:placebo. The primary end point was ≥50% reduction in headaches over a 28-day period. Strikingly, the trial stopped due to futility—60% of participants in all three arms met the primary endpoint, but the adverse effects were noted to be much more frequent in the amitriptyline and topiramate groups. Importantly, during the trial, all the participants had an active co-intervention in terms of lifestyle management—they received advice on lifestyle with constant reinforcement to maintain regular meals, adequate hydration, regular exercise and a regular sleep schedule. They were also advised on optimal acute therapy dosing as well as proper technique on how and when to use it. The working conclusion of this trial is that in prevention of migraine in pediatric populations,
first-line options should have a side effect profile comparable to that of placebo and lifestyle modification is essential. It is important to note that 40% of participants in the trial did not meet the primary end point, and the trial excluded those individuals with continuous headache, medication-overuse headache, and high levels of migraine related disability (PedMIDAS >140).
Discussion
Migraine is a very common, disabling primary headache disorder that occurs in children and adolescents. When diagnosing it in children, it is important to do a thorough history and physical in order to rule out secondary causes. There are certain disorders that affect young children that may be early life manifestations of migraine, and their presence may increase the chances of proper diagnosis in patients. After proper diagnosis, it is essential to counsel patients and families on proper lifestyle habits that can help to control their disorder. When a migraine attack does occur, there are many abortive treatment options that can be presented to patients. It is important to counsel patients on proper use, as well as overuse, as this may contribute to further headaches. When headache is very frequent or contributes to a poor quality of life, prevention therapy is likely necessary. There is currently limited evidence that exists for preventive treatments in children, and much of our practice is extrapolated from adult data. There are more clinical trials underway and while we await these results, it is important to remember that in migraine prevention in children and adolescents, we must always start with the option that has the least side effect profile.












