Migraine prevention medication is recommended for: (1) frequent headaches (generally defined as at least four migraines per month that are associated with some degree of impairment); (2) migraines that significantly interfere with a patient’s daily activities despite acute treatment; (3) migraine with brainstem aura and hemiplegic migraine independent of frequency; (4) contraindication to, treatment failure with, or overuse of acute treatment; and (5) patient preference.1 Individuals with fewer than four migraine days monthly are also candidates for preventive treatment if their migraines do not respond satisfactorily to acute treatment or otherwise cause an unacceptable burden. Prevention should also be considered for patients who are overusing acute therapies (more than 2–3 days weekly) or those who have a suboptimal response to symptomatic treatment, as they are at higher risk for disease progression. The goals of preventive therapy are to reduce migraine frequency and migraine severity, reduce the requirement for acute medications, and improve function and disability.1 This article addresses the current status of medicines used for the prevention of migraine, and a rational approach to prescribing these medications in a new era in migraine prevention.
Current use of migraine prophylaxis
Although preventive treatment has been a mainstay of migraine treatment for decades, it remains vastly underutilized. In the American Migraine Prevalence and Prevention study, 25.7% of surveyed individuals met criteria to offer prevention, of whom 60.0% had severe impairment or required bed rest. Prevention should have been considered in an additional 13.1%, yet only 13.0% of respondents were taking a daily preventive medication.2 Further, of the 43.0% of patients with migraine who had never taken a migraine preventive medication, approximately one-third met the expert guidelines for offering it.
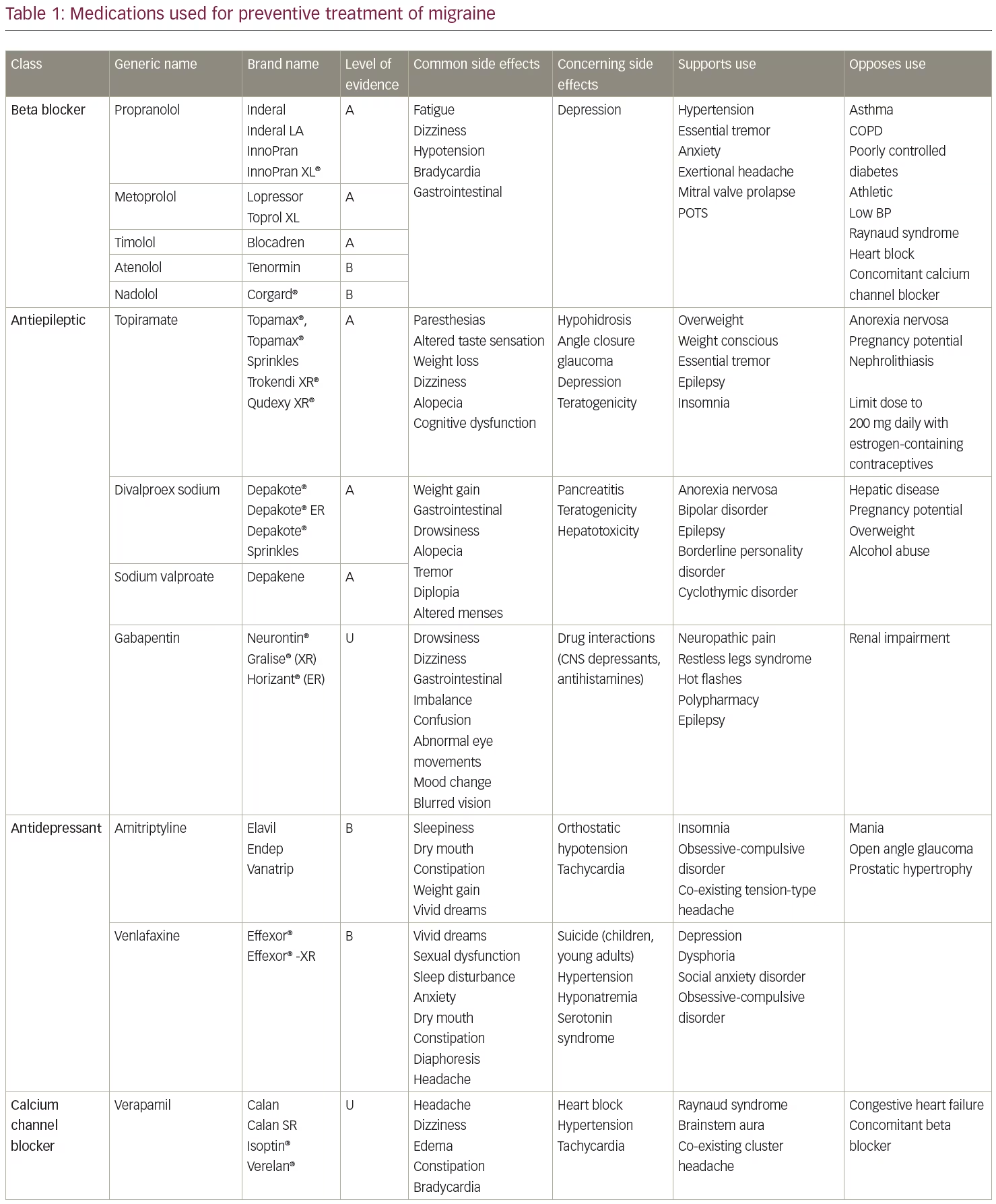
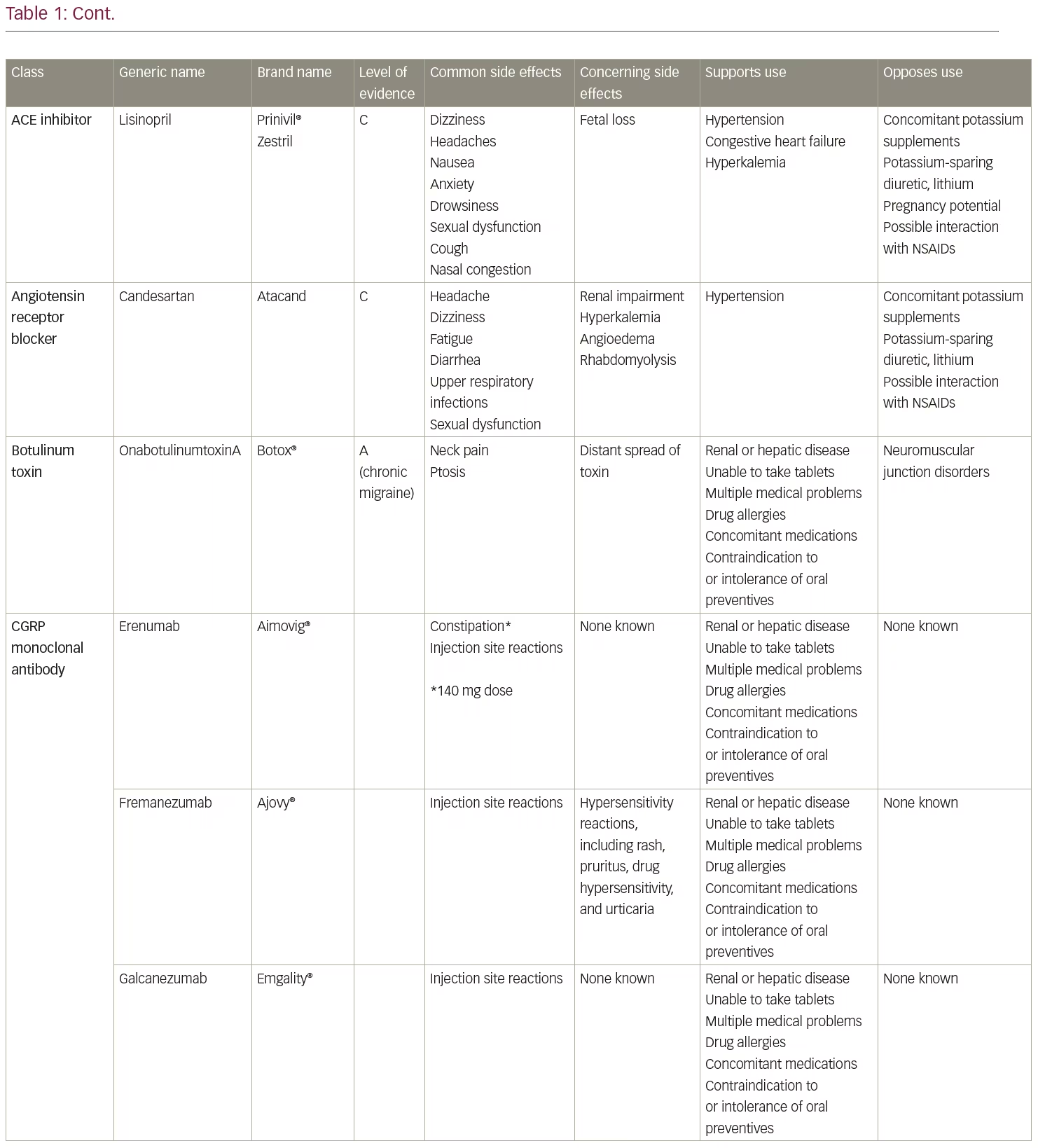
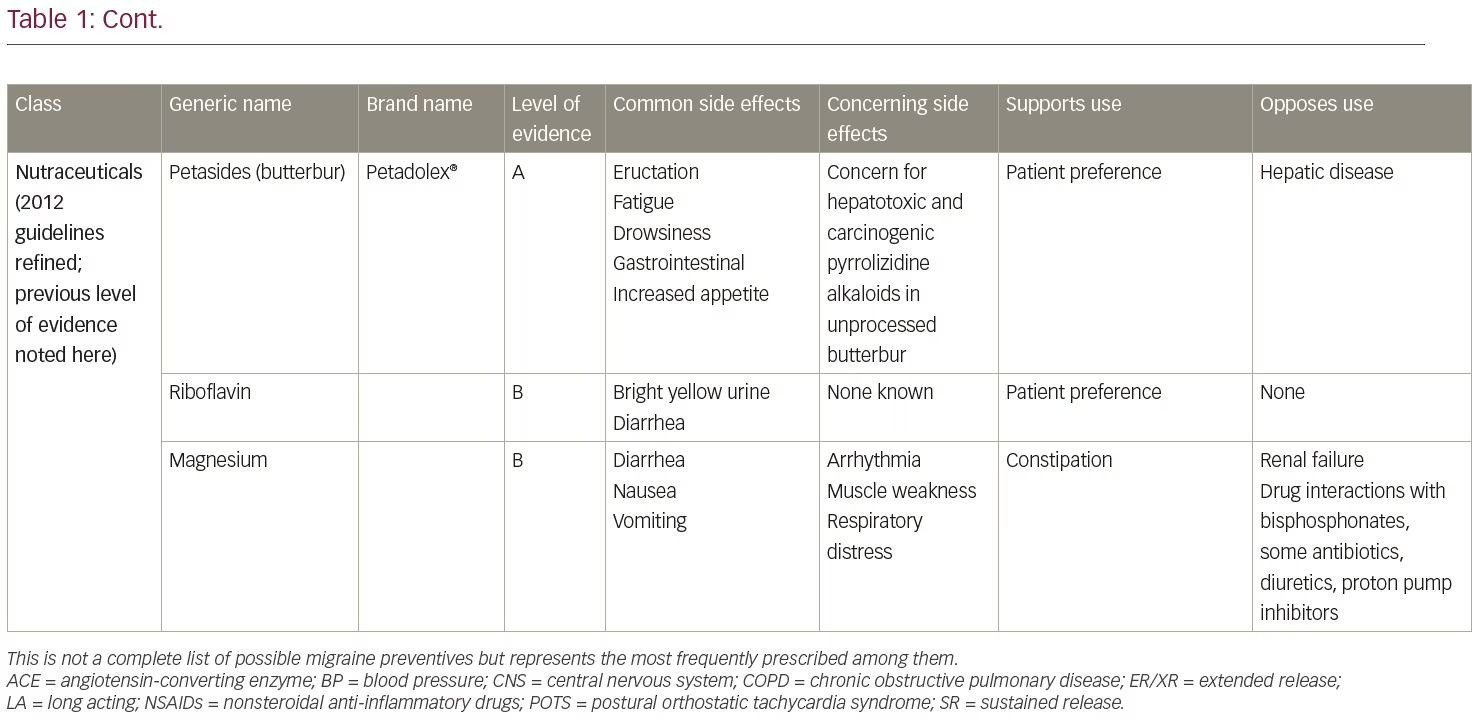
The American Academy of Neurology and American Headache Society published guidelines in 2012 (currently in revision) for preventive treatment selection based on the level of evidence of efficacy in clinical trials.3,4 Medications with the highest level of evidence (Level A) are preferentially recommended as the initial options for migraine prevention. Level A treatments are timolol, propranolol, metoprolol, sodium valproate/divalproex sodium, topiramate, and petasites (butterbur). However, petasites preparations may contain pyrrolizidine alkaloids, which are associated with liver toxicity and are no longer recommended due to long-term safety concerns. Other treatments with Level A evidence include onabotulinumtoxinA for the treatment of chronic migraine and frovatriptan for short-term prevention of menstrual migraine. Of these, topiramate, timolol, sodium valproate/divalproex sodium, and onabotulinumtoxinA are Food and Drug Administration (FDA)-approved for migraine prevention. Monoclonal antibodies administered subcutaneously to target calcitonin gene-related peptide (CGRP) (fremanezumab, galcanezumab) or its receptor (erenumab) were first FDA-approved for migraine prevention in May 2018, meeting current standards for Level A efficacy.5,6 A fourth, eptinezumab, also meets level A standards and is pending FDA approval for intravenous administration. The Canadian Guidelines, based on medical literature and expert consensus, also include a strong recommendation for candesartan and gabapentin among those prescription drugs appropriate for migraine prevention.7
Until recently, preventive medications used for migraine treatment were initially developed for other conditions and later found to be useful for migraine prevention, either discovered by serendipity or suspected based on their mechanisms of action vis-à-vis a more sophisticated understanding of migraine pathophysiology over time. These agents typically act on multiple targets involved in the migraine process, such as serotonin receptors, calcium receptors, gamma-aminobutyric acid (GABA), norepinephrine, CGRP, sodium channels, and others.8 In order to increase their tolerability, they are started at low doses and gradually increased to the target dose according to individual response; i.e., the lowest dose at which the patient achieves the maximum benefit. An adequate trial of preventive medication is at least 2 months at the maximally tolerated or target dose. However, many preventives confer additional benefit when taken for ≥6 months. ‘Success’ is currently defined as a ≥50% reduction in migraine days compared to pre-treatment frequency.9 A 50% reduction in migraine days, while welcome, still leaves a substantial migraine-related burden for many patients. If monotherapy does not yield desired results, combining preventive therapies affords the potential to raise the bar with a goal of total or near-complete headache freedom.
Just as triptans were developed as specific acute migraine medications, the aforementioned monoclonal antibodies specifically targeting CGRP or its receptor herald an era of medications designed for the prevention of migraine.5,6 Erenumab, fremanezumab, galcanezumab, and eptinezumab all have at least one large phase III, randomized clinical trial demonstrating statistical superiority for migraine prevention over placebo.5,6,10 Small molecules targeting CGRP, the ‘gepants’, are also being developed as oral medications; rimegepant and ubrogepant have completed phase III clinical trials for acute treatment, and atogepant phase III studies for prevention are underway (information on various studies available at ClinicalTrials.gov).10 In contrast to the traditional oral migraine preventives, treatment with CGRP antagonists is initiated at an effective dose without titration. Therapies directed at other specific components of migraine physiology, such as pituitary adenylate cyclase activating polypeptide and acid-sensing ion channels, have promise as additional options to treat this complex disorder.11–13
Medications with less rigorous evidence are used off label for migraine prevention. In some cases, they are older medications that were FDA-approved for other indications and marketed prior to the requirement for large, randomized, placebo-controlled trials that are the standard by which new treatments are tested to receive an FDA indication for migraine prevention. Other medications have less robust levels of evidence based on small studies or case series, or have not been formally studied for migraine prevention but have gained acceptance based on clinical experience. Some medications with lower levels of evidence are preferred in special circumstances, such as flunarizine (not available in the USA), acetazolamide for the prevention of hemiplegic migraine, and verapamil for migraine with brainstem aura;14 to date, both migraine types have been systematically excluded from clinical trials of migraine preventives, possibly based on erroneous assumptions of their pathophysiology from older (vascular) theories of migraine mechanisms.
In addition to level of evidence, other considerations affect the selection of a preventive medication in an individual patient, making the treatment strategy somewhat of an art form. Co-existing medical conditions, concomitant medications, allergies, cost and insurance coverage, pregnancy potential, possible side effects (which may be advantageous or have a negative impact), route of administration, time to achieve maximal effectiveness, and preference for self-administration or office administration all factor into the decision.9 Given that more frequent daily dosing is associated with poorer adherence to therapy, the dosing interval is another important consideration.14–16 Patients with more frequent and disabling migraines, or those who also have other headache types, may require more than one preventive agent for optimal effectiveness. Strict adherence to the guidelines is problematic for treating patients aged >65 years, as they are typically excluded from migraine prevention trials. Similarly, most of the current preventive medications have not been studied in the paediatric population. Fortunately, the FDA now requires that medications approved for adult use also be tested in children when possible.
Migraine prophylactic treatments
As noted above, there are an expanding number of migraine preventive options and complexities that may arise in choosing preventive agents (Table 1). Oral medications remain the first-line therapies for migraine prevention in most circumstances. In terms of efficacy in preventing migraines, FDA-approved medications confer similar reductions in migraine frequency. For instance, clinical trials demonstrate approximately 46% of patients given 100 mg/day topiramate, compared with 23% of patients given placebo, achieved ≥50% reduction in monthly migraine frequency.17,18 Topiramate, a frequently prescribed preventive medication in the USA, was also studied in chronic migraine, with two studies showing effectiveness for up to 16 weeks of follow-up.17–19 Valproic acid reduced mean monthly migraine rates by 30–40%, compared to baseline, with no dose-response relationship.20 Amongst the tricyclic antidepressants, amitriptyline is the best studied with two randomized trials showing it to be more likely than placebo to produce a 50% reduction in episodic migraine frequency.19 In a comparative effectiveness meta-analysis of trials of different prophylactic migraine medications (both FDA-approved and unapproved medications in a mostly episodic migraine population), the efficacy of most drugs were found to be similar to one another.19
Regarding medications that are parenterally administered, clinical studies of erenumab, the first FDA-approved monoclonal antibody targeting the CGRP receptor, demonstrated approximately a 40% reduction in mean monthly migraine days compared to baseline in both episodic and chronic migraine, with 30–40% of participants having at least a 50% reduction in monthly migraine days during the 12-week study period.21,22 The other CGRP monoclonal antibodies (galcanezumab, fremanezumab, eptinezumab) have shown similar efficacy to erenumab.23–27 Almost 70% of patients achieved a 50% reduction in headache days at 56 weeks in the pooled analysis of onabotulinumtoxinA for chronic migraine, illustrating that improved effectiveness over time is common with preventive treatments.28 Given that the medications with the best evidence have similar efficacy in clinical trials, other distinguishing characteristics are helpful in selecting a preventive therapy for a given patient.
Factors influencing the selection of a preventive medication
Frequency and route of administration
Numerous studies confirm that adherence to a daily regimen of oral medications is inversely related to dosing frequency.15,16 For those preventive treatments with a long half-life (e.g., tricyclic antidepressants), once-daily dosing is easily achieved. Many migraine preventives, which were initially marketed with two to three times daily dosing, have been reformulated into extended or sustained release preparations that can be dosed once daily (e.g., gabapentin, topiramate, valproic acid, propranolol).29 Improved adherence with once-daily dosing increases the likelihood of improved effectiveness and reduced healthcare resource use (e.g., emergency department visits and less reliance on acute rescue medication).30
A few medications are available as a liquid or suspension for those patients who are unable to swallow pills or require a liquid for other medical reasons. Nortriptyline, valproic acid, cyproheptadine, and propranolol are supplied as liquid suspensions and topiramate and valproic acid are formulated as sprinkle capsules.31
For patients who are willing to consider parenteral therapies, onabotulinumtoxinA (indicated for chronic migraine) and the subcutaneous and infused CGRP monoclonal antibodies offer the benefit of infrequent administration. The in-office administration of onabotulinumtoxinA and infused treatments may improve adherence, and gives the provider the assurance that the patient has received the treatment as expected.
Co-existing conditions
Selection of a preventive medication must take into account the potential for improvement or worsening of a co-existing condition. Conditions are considered to be comorbid with migraine in that they occur more frequently in migraine patients than in the general population. Those confirmed in population studies include anxiety/phobias, panic disorder, depression, bipolar disorder, Raynaud syndrome, epilepsy, asthma, and non-headache pain, such as fibromyalgia.9,32–35 Other disorders that are generally considered to be comorbid but not confirmed in population studies include patent foramen ovale, benign positional vertigo, mitral valve prolapse, restless legs syndrome, systemic lupus erythematosus, irritable bowel syndrome, essential tremor, post-traumatic stress disorder, cerebral artery dissection, and obsessive-compulsive disorder. Patients with migraine may also have other types of headaches such as tension-type headache, primary stabbing headache or cluster headache.
Migraine is also an independent risk factor for various cardiovascular conditions, such as ischemic stroke and myocardial infarction, and is also associated with a higher rate of suicide.35,36 In addition to migraine-related conditions, patients may have other medical problems that could potentially be influenced by a migraine preventive treatment. As oral medications are either metabolized by the liver or excreted through the kidney, hepatic and renal disease affects medication choices.
Pregnancy considerations
Migraine affects women three times more commonly than men, often begins around menarche and is most prevalent during the reproductive years. It may improve during the second trimester of pregnancy. No medications for the prevention of migraine have been studied during pregnancy. Sodium valproate/valproic acid carries a boxed warning in its label about use during pregnancy and topiramate has a warning and precaution in its label about its use during pregnancy because of their teratogenic potential and should be avoided in women who have plans to conceive. Sexually active females with pregnancy potential who are already taking these medications should be counseled appropriately.20,37–39 Otherwise, whether or not to initiate or continue a preventive medication during pregnancy involves weighing the risks and benefits of therapy, patient preference, and obstetrical considerations.
Concomitant medications
The addition of any new medication to a patient’s regimen requires a careful evaluation for possible drug interactions.
Side effect profile
Patients are, understandably, highly concerned about potential medication side effects. This factor contributes to the reluctance of some patients to try a preventive treatment, the underutilization of prescribed medications, and certainly the high discontinuation rate of migraine preventive medications. It is not possible to predict who will experience side effects or which side effect a given person may have. Thus, a discussion of possible side effects, emphasizing the common and most dangerous ones, is imperative. However, side effects can also work to a patient’s advantage. For example, topiramate is a good choice for someone who is overweight or concerned about weight gain, whereas sodium valproate/valproic acid or a tricyclic antidepressant often cause weight gain. Beta blockers are not the drugs of choice for physically active patients but may help someone with anxiety. As the parenteral CGRP therapies were only introduced in clinical practice recently, their overall safety profile in the general population is still uncertain, although injection site reactions, constipation, cramps, muscle spasms, and hypersensitivity reactions have all been reported during the clinical trials of these medications. Starting oral preventive medications at a low dose and gradually increasing to the target therapeutic dose is the recommended strategy to minimize side effects. Additionally, the pharmacokinetic profile of extended- and sustained-release preparations (e.g., topiramate, valproate, propranolol) often improve tolerability.19,29
Polypharmacy
There is no one panacea when it comes to migraine preventive drugs. Response rates and a patient’s tolerability to treatment will vary. Migraine has a complex pathophysiology that includes both central and peripheral mechanisms, and the compounds available for migraine prophylaxis do not target all the sites and mechanisms involved in migraine attacks. When patients partially respond to a single treatment, or cannot tolerate recommended doses of therapies, combinations of migraine preventatives that target different aspects of the pathophysiology of migraines may be beneficial.13 For example, topiramate-based medications, which act centrally, may be combined with peripheral-acting agents, such as onabotulinumtoxinA or an anti-CGRP monoclonal antibody. Although polypharmacy is commonly employed with benefit in clinical practice, there are little data regarding its effectiveness as FDA registration studies traditionally evaluated monotherapy. However, one CGRP antagonist clinical trial to date (fremanezumab) included participants taking a stable dose of one or more other oral migraine preventive medications.10,24,25
Access to treatment
Despite the provider’s best judgment and medical opinion, cost and coverage often determine which medications a patient has access to. Whether the product is generic or branded may have little relevance in this regard, depending on contractual arrangements in the payer’s formulary. Sometimes, even after submitting appeals and letters of medical necessity, medications that will likely benefit the patient are denied, and the patient is unable to afford them. Step-edits imposed by insurers often preclude using FDA-approved treatments with high levels of medical evidence as first-line therapies until others are tried first; ironically, covered treatments generally include medications with little or no evidence supporting their efficacy in migraine prevention.40 Moreover, despite the paucity of board-certified headache medicine specialists (fewer than 600) in the United States, some states have imposed limitations on who is authorized to prescribe the anti-CGRP monoclonal antibodies, denying coverage for prescriptions from neurologists, pain management specialists, and advance practice providers. Given that there are states with no board-certified headache medicine specialists, this effectively prohibits access to millions of people who would otherwise be candidates for these therapies.
Some patients using onabotulinumtoxinA who have experienced improvement but still have significant headache-related disability are being forced by payers to discontinue onabotulinumtoxinA prior to knowing whether or not an anti-CGRP monoclonal antibody is beneficial or tolerated. Others who have enjoyed marked improvement with onabotulinumtoxinA and an anti-CGRP monoclonal antibody are being denied coverage for either therapy citing lack of evidence in clinical trials for this particular combination therapy; these policies conflict with the American Headache Society’s position statement on incorporating new migraine treatments into clinical practice.41
Rational prescribing
The previously accepted standard for improvement has been at least a 50% improvement in headache frequency and/or severity. However, it is possible to achieve greater than 75% improvement with either monotherapy or combination therapy. Table 1 summarizes commonly used preventive medications and factors to guide decision making. Although the common dogma is that providers be very familiar with two or three medications and use them for a given condition, the wide variety of migraine preventive treatments sometimes requires that clinicians expand their therapeutic horizons.
A prudent prescribing strategy is to only change one medication or dosage at a time, in order to accurately assess effectiveness and tolerability. Thus, when changing preventive medications, it is rational to start the additional preventive without changing the patient’s current regimen, unless the current preventive is causing intolerable side effects, worsening of headaches, or poses the potential for a drug-drug interaction. After ensuring that the patient tolerates the new medication, the initial preventive drug can be withdrawn if there is no synergic benefit.
Medication efficacy and safety are the foundations of rational prescribing decisions, but migraine treatment can be maximized if patient characteristics and preferences are also included within this framework. Because of the complex pathophysiology of migraines, a clinician may need to employ multiple strategies, including lifestyle modifications and psychological therapies, to effectively treat their patient.42 The likelihood of a migraine attack may be reduced by targeting multiple aspects of the migraine pathway, rather than by targeting just one. Although one goal of preventive migraine treatment is to reduce the reliance on acute medications, rescue medications may be needed. It is important to counsel the patient on the use of rescue medications to decrease the likelihood of complications (e.g., medication overuse headache).
Conclusion
Although the advent of new migraine preventives entering the marketplace offers opportunities for improved migraine control for patients that have not achieved ideal benefit from existing treatments, the long-term safety of these medications has yet to be confirmed in clinical practice; although open-label extension studies are encouraging thus far.43–45 Third-party coverage typically dictates the sequence and breadth of introducing new therapies, regardless of a patient’s expectations or preferences. It is important to note that foundational medications in this therapeutic arena that have served many patients well for decades still have a prominent role in migraine prevention. With all the pieces in place, rational prescribing decisions for migraine prophylaxis can be accomplished.












