Although preventive treatment has been a mainstay of migraine treatment for decades, it still remains vastly underutilized.1,2 In recent years, however, the profusion of new treatments for migraine has expanded the therapeutic arsenal for the preventive treatment of migraine.
Clinical care pathway for the prevention of migraine
John Rothrock
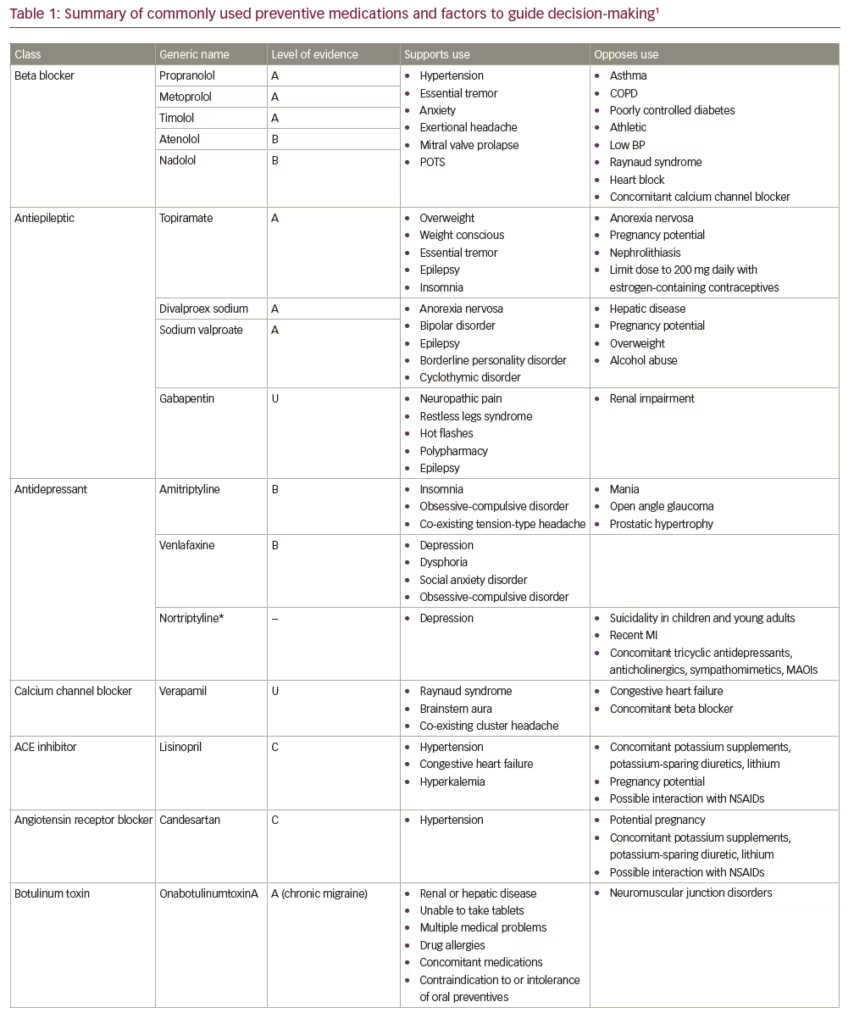
When selecting the appropriate preventive therapy, key factors that the healthcare provider needs to consider are (i) any experience the patient has had with prophylactic therapy in the past, (ii) the presence of any comorbidities that might influence the choice of treatment, and (iii) the patient’s current migraine subtype (most importantly, whether the patient suffers from episodic or chronic migraine). Current guidelines recommend considering preventive treatments for people who experience frequent and disabling migraine headaches, and those suffering headaches on ≥4 days per month with normal functioning.2 Of the available preventive medications, and dependent to a large degree on requirements mandated by insurers, most first-line therapies for migraine prevention are oral medications. The oral therapy chosen should have a solid base of evidence for its use in migraine and should be started at a low dose, which is sequentially advanced to the target therapeutic dose so as to minimize the risk of any side effects.1
To effectively treat the patient, and especially a patient with chronic migraine, the clinician may need to employ a “holistic” strategy. Such management extends beyond traditional pharmacotherapy to include attention to co-morbid medical disorders that may aggravate migraine (e.g., obesity, depression), encouraging lifestyle modifications (improved sleep hygiene, initiation of a diet or exercise/aerobic conditioning program) and use of adjunctive therapies such as vitamins (riboflavin), supplements (Coenzyme Q10, petasites) or neuromodulation devices (e.g., transcutaneous supraorbital neurostimulation) for migraine prophylaxis.3
A change of treatment should be considered if there are tolerability issues, contraindications that arise and/or insufficient efficacy. To accurately assess effectiveness and tolerability, a prudent prescribing strategy is to change only one medication or dosage at a time when altering a preventive regimen.1 Typically, it is best to start additional preventive therapy without changing the patient’s current regimen (assuming there are no issues involving tolerability, drug–drug interactions or adverse effects on efficacy).1 In an effort to determine whether the two agents taken together are acting in synergy or the patient is simply exhibiting a positive response consequent to the second agent alone, the preventative therapy initially prescribed can be tapered down or withdrawn and the patient’s clinical response correspondingly observed.1 In acknowledgment of migraine’s complex pathophysiology, combining preventive therapies that appear to possess different mechanisms of action may be a viable option if monotherapy does not yield a sufficiently positive clinical response.1,4
First-line therapies recommended by the current American Academy of Neurology/American Headache Society (AAN/AHS) guidelines include antiepileptic drugs (divalproex sodium or topiramate) and beta blockers (metoprolol, propranolol or timolol) (Table 1).5,6 Also, considering the clinical evidence base, the next iteration of the guidelines is expected to accommodate the recently-approved calcitonin gene-related peptide (CGRP) monoclonal antibodies.7–11 Generally speaking, medications that have the most compelling evidence base have demonstrated similar efficacy in clinical trials for the prevention of migraine. As such, to ensure optimal safety, efficacy, tolerability and adherence, whatever prophylactic therapy is prescribed should be appropriate to the specific clinical circumstances and “customized” so as to meet the needs and satisfaction of the individual patient.
Identifying the right patient for the right treatment
Ira Turner
The challenge in the prevention of migraine in the individual patient is finding the correct balance between treatment effect, side effects and concomitant medications. In particular, consideration must be given to any co-morbid conditions the patient may have. As oral medications are either metabolized by the liver or excreted through the kidney, concomitant medications, as well as co-morbid conditions (e.g., obesity, hypertension, hepatic and renal diseases), may affect medication efficacy and safety. For example, topiramate may be a good choice for someone who is overweight or concerned about weight gain, whereas sodium valproate/valproic acid or a tricyclic antidepressant often cause weight gain, and beta blockers are not the drugs of choice for physically active patients, but may help someone with anxiety.1 Similarly, although migraine affects women three times more commonly than men, no medications for the prevention of migraine have been studied during pregnancy. Indeed, topiramate and sodium valproate/valproic acid carry warnings about potential teratogenic properties and should therefore be avoided during pregnancy.12,13
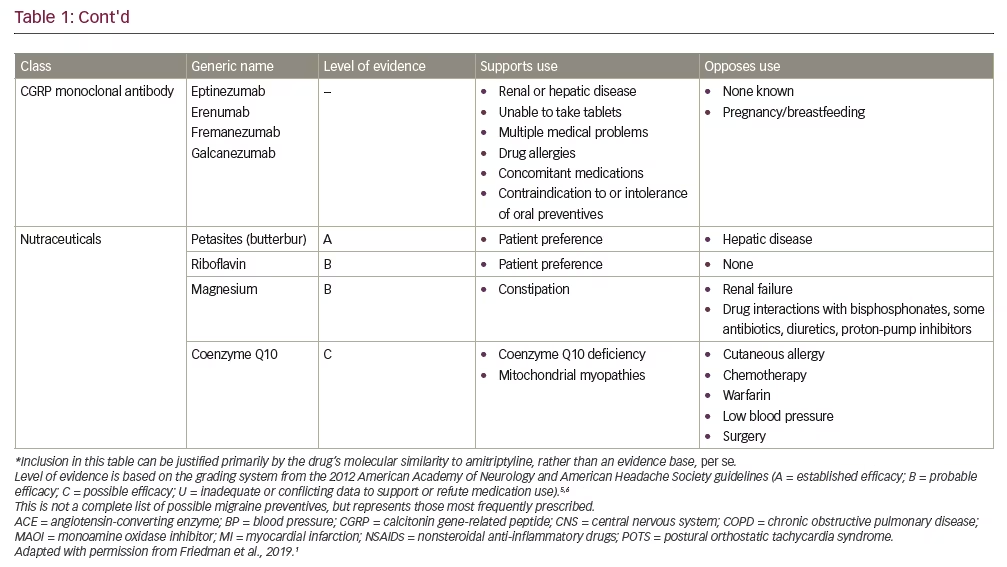
The medication formulation is also an important consideration. While most oral medications are available in the form of tablets or capsules, some are also available as a liquid or suspension for those patients who are unable to swallow pills or require a liquid for other medical reasons.1,14 Different formulations may also have different properties that may benefit specific patients. For example, the pharmacokinetic profile of extended- and sustained-release preparations (e.g., topiramate, valproate, propranolol) may also improve tolerability. Further, numerous studies confirm that adherence to medication is inversely related to dosing frequency.15,16 As such, once-daily dosing with extended/sustained-release formulations may be the optimal choice in many patients. For patients who are willing to consider parenteral therapies, onabotulinumtoxinA (indicated for chronic migraine) and the recently approved subcutaneously infused CGRP monoclonal antibodies (mAbs: eptinezumab, erenumab, fremanezumab, galcanezumab) also offer the benefit of infrequent administration, with injections administered monthly or even quarterly.7–11
The spectrum of choices for pharmacologic prophylaxis of migraine includes medications first utilized for that purpose in the 1960s (e.g., amitriptyline) to the anti-CGRP mAb “designer drugs” that emerged in 2018. What reliable data we possess concerning the relative efficacy of these medications does not suggest a clear hierarchy. Thus a wide variety of considerations, from patient preference and characteristics, through co-morbid conditions and concomitant medications, to specific aspects of the preventive therapies themselves, should be taken into account to provide a global assessment of the specific clinical circumstances and so enable the optimal treatment to be selected for the individual patient.
Measuring the effectiveness of therapy
Jan Lewis Brandes
“Success” with regard to migraine therapy, as currently defined in clinical trials and by the US Food and Drug Administration (FDA), includes a reduction in mean migraine days, along with a ≥50% reduction in migraine days within 3 months, compared with pre-treatment frequency (typically measured using a patient diary).17 While these reductions in migraine duration and frequency are important to patients, even a 50% reduction in migraine days still leaves a substantial migraine-related burden for many patients. Success in preventive therapy from patient and physician perspectives usually includes more than just clinical trial primary endpoints, such as an overall reduction in migraine days, reduction in the duration and severity of attacks, improvement in both the speed and efficacy of acute migraine and non-migraine-specific medications, improvement in the ability to function on migraine days, and importantly, tolerability to any new preventive medication.
Health-related quality of life (HRQoL) is increasingly becoming a key factor in the assessment of treatment success in migraine.17 Particularly for the patient, frequency of headaches may represent only one aspect of treatment success, and not necessarily the primary goal, if they also experience a substantial reduction in headache severity and the disability associated with attacks. Importantly, as patients improve, they may become increasingly anxious when a breakthrough attack does occur. Once the patient becomes accustomed to longer migraine-free intervals, any “new” attack may seem more provoking and disturbing, serving to remind them of their previous migraine disability before effective treatment, and causing distress. As such, the assessment of treatment success in the prevention of migraine should go beyond a simple “migraine day” diary and incorporate measures of HRQoL that reflect global components of migraine disability, perhaps using other tools such as the migraine disability assessment questionnaire (MIDAS),18 or the migraine-specific quality of life questionnaire (MSQ).19 Currently, no single instrument for the measurement of migraine’s burden encompasses all aspects of that burden: the frequency and severity of headaches, the impact on mood, migraine disability (e.g., days of dysfunction/function), and HRQoL.
An objective measure of a patient’s headache burden at the start of treatment, allowing for close monitoring of the patient’s progression over time and for medication adjustments as needed, together with subjective quality of life parameters, would be a valuable method of assessing treatment response—both for migraineurs and those who treat them. 












