Levodopa (l-dopa) remains the most effective therapy for Parkinson’s disease (PD), but unfortunately after 3–5 years of treatment it is associated with the appearance of motor complications: motor and non-motor fluctuations (e.g. on–off phenomena, end-of-dose wearing off) and dyskinesias.1 Many non-dopaminergic transmitters, such as glutamate, have been implicated in the neural mechanisms underlying the development of these complications.
Pain is an important non-motor symptom of PD often underestimated and inadequately treated. According to epidemiological studies, the prevalence of chronic pain in PD patients oscillates from 30 % to 85 %2,3 and it has been shown to be associated with impaired sleep, depressed mood and reduced health-related quality of life and, in some patients, can be the dominant symptom of PD.4 The neurobiology of pain in PD is complex and appears to involve glutamatergic and other neurotransmitter systems in addition to the dopaminergic one.5
Safinamide is structurally unrelated to any other drug for the treatment of PD. It acts, in fact, through a new multimodal mechanism of action (dopaminergic and non-dopaminergic) that includes the enhancement of the dopaminergic transmission through the selective, reversible inhibition of the monoamine oxidase-B (MAO-B) enzyme, and the control of the neuronal excitability through the state-dependent blockade of the voltage-gated sodium channels: it has a preferential interaction with the inactivated sodium channels and prevents their recovery to the active state, with the consequent modulation of the glutamate release. Safinamide also modulates N-type calcium channels without affecting L-type calcium channels (no effects on blood pressure or heart rate). Moreover, safinamide is well tolerated with a favourable side-effect profile, has no major drug–drug interactions and no diet restrictions.6,7
Safinamide 50 and 100 mg/day have been approved by the European Medicines Agency for the treatment of adult patients with idiopathic PD as add-on therapy to a stable dose of l-dopa alone or in combination with other PD medicinal products in mid- to late-stage fluctuating patients.
Pivotal Studies
Studies 0168 and SETTLE9 were phase III, multicentre, randomised, double-blind, placebo-controlled, parallel-group, 24-week duration trials designed to evaluate the efficacy and safety of safinamide versus placebo as add-on therapy to a stable dose of l-dopa (alone or with other antiparkinsonian drugs) in patients with mid- to late-stage PD and motor fluctuations. The primary efficacy measure was the change from baseline to 24 weeks in daily ON time with no/non-troublesome dyskinesia as recorded by patients in an 18-hour diary. In both studies, the addition of once-daily oral safinamide, at the doses of 50–100 mg (016 study) or 100 mg (SETTLE study), significantly increased ON time with no/non-troublesome dyskinesia.

In study 016, at week 24, there were significant differences in the least squares (LS) mean change versus placebo in both the safinamide 50 mg/day (0.51 hours; 95 % confidence interval [CI] 0.07–0.94; p=0.0223) and the safinamide 100 mg/day (0.55 hours; 95 % CI 0.12–0.99; p=0.0130) groups.
In the SETTLE study, the LS mean change of safinamide 100 mg/day versus placebo was significantly different (0.96 hours; 95 % CI 0.56–1.37; p<0.001).
These results suggest that safinamide improves motor symptoms without increasing the risk of developing dyskinesia or worsening troublesome dyskinesia.
Study 01810 was the long-term extension (up to 24 months) of study 016. The primary endpoint was the mean change from baseline (study 016 start) to study end (2 years) in the total Dyskinesia Rating Scale (DRS) score evaluated during ON time.
The main secondary efficacy endpoint was the change in ‘ON’ time without troublesome dyskinesia. After 2 years, there was no significant difference in the primary endpoint between safinamide and placebo, despite a decrease from baseline in mean DRS score of 31 % and 27 % with safinamide 50 and 100 mg/day, respectively, compared with a decrease of only 3 % with placebo. Regarding the ON time with no/ non-troublesome dyskinesia, the improvements seen at 6 months were still present at the end of the trial, showing that safinamide maintained the benefits in the long term. The LS mean change versus placebo was 0.67 hours for the safinamide 50 mg/day group (95 % CI 0.23, 1.11; p=0.0031) and 0.83 hours for the safinamide 100 mg/day group (95 % CI 0.39, 1.27; p=0.0002).
The incidences of treatment-emergent adverse events (TEAEs), drugrelated adverse events, discontinuations due to TEAEs and serious adverse events were similar in the safinamide and placebo groups in both the 016 and SETTLE studies. The majority of AEs were rated as mild or moderate. Common side effects (reported in ≥5 % of patients in any group) included worsening of PD, cataract, back pain, pyrexia, dyskinesia and hypertension. The incidences of TEAEs, both newly emergent and re-emergent, in the combined 016 and 018 study population were also similar across groups; however, newly emergent TEAEs were reported in significantly fewer safinamide 50 or 100 mg recipients compared with placebo during the 018 extension study (76.7 and 78.3 versus 85.1 %; p=0.0329). No dose response for safinamide on the frequency of AEs has been reported.
Post Hoc Analyses Motor Fluctuations
In a first post hoc analysis,11 data of studies 016 and SETTLE were pooled to assess the changes from baseline in ON time (with no or nontroublesome dyskinesia) and OFF time in subgroups of patients who were receiving only l-dopa at baseline, who were classified as ‘mild fluctuators’ (daily OFF time ≤4 hours) and who were receiving concomitant dopamine agonists (DA-ago) or catechol-O-methyltransferase (COMT) inhibitors, and to assess the effects of safinamide versus placebo on cardinal PD symptoms (Unified Parkinson’s Disease Rating Scale [UPDRS] score of bradykinesia, rigidity, tremor and gait) during ON time.
Statistical Methods
Comparisons of the mean changes from baseline to week 24 for the active treatment group relative to placebo were performed using linear effects models with treatment group and study index as fixed dummy effects and baseline value as continuous covariate (analysis of covariance [ANCOVA] analyses). The intention-to-treat patient populations were used for all post hoc analyses, while the last observation carried-forward approach was applied to account for missing data at study termination. No p value adjustments were made for multiplicity generated by secondary and subgroup analyses. All statistical analyses were performed using SAS software version 9.4.
Results
The pooled data set for studies 016 and SETTLE comprised 971 patients who received safinamide 100 mg once daily (n=487) or placebo (n=484). The results pertaining to the changes in ON time (with no or nontroublesome dyskinesia) are reported in Table 1, while those related to OFF time are shown in Table 2.
Safinamide 100 mg/day significantly increased the mean ON time (with no or non-troublesome dyskinesia) and reduced the mean OFF time when used as first adjunct therapy in l-dopa-treated patients:



the increase in ON time was 1.49 hours (95 % CI 0.79, 2.19), compared with 0.33 hours with placebo (95 % CI -0.34, 1.01; p=0.0206), while the decrease in OFF time was -1.59 hours (95 % CI -2.21, -0.97) compared with -0.23 hours with placebo (95 % CI -0.83, 0.36; p=0.0024). Similar results were obtained in patients with mild motor fluctuations: safinamide 100 mg/day significantly increased the daily ON time of 0.94 hours (95 % CI 0.59, 1.30), as opposed to 0.22 hours with placebo (95 % CI -0.15, 0.60; p=0.0062), and decreased the daily OFF time of -0.74 hours (95 % CI -1.01, -1.47), as opposed to -0.10 hours with placebo (95 % CI -0.39, -0.18; p=0.0018).
The mean daily ON time (with no or non-troublesome dyskinesia) and OFF time were favourably changed, compared with placebo, to similar extents regardless of whether patients were receiving concomitant DA-ago or COMT inhibitors. For the patients already on DA-ago, safinamide 100 mg/day increased the mean daily ON time (1.47 hours; 95 % CI 1.20, 1.73) significantly more than placebo (0.60 hours; 95 % CI 0.34, 0.87; p<0.0001) and reduced the OFF time of -1.49 hours (95 % CI -1.73, -1.25) compared with -0.65 hours with placebo (95 % CI -0.90, -0.41; p<0.0001). In the subgroup of patients taking stable doses of a COMT inhibitor in addition to l-dopa, safinamide 100 mg/day increased the mean daily ON time (1.52 hours; 95 % CI 1.20, 1.83) significantly more than placebo (0.74 hours; 95 % CI 0.42, 1.05; p=0.0007) and decreased the OFF time of -1.54 hours (95 % CI -1.82, -1.25) compared with -0.74 hours with placebo (95 % CI -1.02, -0.45; p<0.0001).
Additionally, safinamide improved the scores of bradykinesia (p=0.0102), rigidity (p=0.0006), tremor (p=0.0001) and gait (p=0.0118) (see Table 3).
Dyskinesias
A first post hoc analysis of the changes in DRS scores was already published in the 018 study: considering only patients with moderate-tosevere dyskinesia at baseline (DRS total score >4), the mean change in DRS scores from baseline to study end was significantly different in the safinamide 100 mg/day group compared with placebo.10
As the mean changes in evaluation may be affected by a bias if the distribution is skewed, a second post hoc analysis12 was performed evaluating the categorical changes in DRS scores at the end of study 018 after stratifying patients based on the presence or absence of dyskinesia (DRS score >0 or DRS score =0, respectively) at baseline, and by additional subgroups based on whether the dose of l-dopa had been changed during the entire treatment period of 24 months.

Statistical Methods
Comparisons of active treatments (safinamide 100 mg and safinamide 50 mg) versus placebo were performed using the Wilcoxon rank-sum test for independent samples. Since in the original protocol of study 018 the multiplicity issue (over treatment groups) was handled by using a prespecified ‘sequence of comparisons’ approach, no adjustment of type 1 error was needed for multiple comparisons. All statistical analyses were performed using SAS software version 9.4.
Results
For the overall treated population (with or without dyskinesia at baseline), considering patients with no changes in l-dopa dose during the study, safinamide 100 mg/day significantly increased the proportion of patients with improvement in the DRS score compared with placebo (p=0.0488). Conversely, the proportion of patients who had worsening of DRS scores was greater in the placebo group. This suggests that the beneficial effect of safinamide 100 mg on DRS scores was not dependent on changes in l-dopa dose, as this analysis excluded patients with an l-dopa dose reduction (see Figure 1).
In the subgroup of patients with dyskinesia at baseline, safinamide 100 mg/day significantly increased the proportion of patients with improvement in the DRS score versus placebo (p=0.0153) (see Figure 2), while in the same subgroup with no changes in l-dopa dose the proportion of patients with improvements was nearly significant (p<0.0546) (see Figure 3).
Safinamide 50 mg/day showed intermediate results generally better than placebo even if the statistical significance was not reached.
Pain
A post hoc analysis of pooled data from the 016 and SETTLE trials was performed to evaluate the effects of safinamide versus placebo on the reduction of concomitant pain treatments and on the scores of painrelated items of the Parkinson’s Disease Quality of Life Questionnaire (PDQ-39) ‘Bodily discomfort’ domain.13
The variables assessed were as follows:
- Proportions of patients not using pain treatments during the study
period, and the number of pain treatments used in the group
receiving safinamide 100 mg/day versus the placebo group (pain
treatments included analgesics – anatomical therapeutic chemical
[ATC] drug class N02, anti-inflammatory and antirheumatic products
[ATC drug class M01] and topical products for joint and muscular
pain [ATC drug class M02]). - Scores for items 37–39 of the PDQ-39 questionnaire, one of the most
widely and specific scales used in PD for the assessment of the
quality of life and both the motor and non-motor symptoms of patients.
These items are related to ‘Bodily discomfort’ (37, painful cramps
or spasm; 38, pain in joints or body; 39, unpleasantly hot or cold).
Statistical Methods
Comparison of percentage of patients not using concomitant pain drugs after 6 months of treatment was performed using conventional Pearson’s chi-square.
The reduction in the number of analgesic treatments associated with safinamide 100 mg/day versus placebo was estimated by means of a negative binomial regression model obtained with a generalised linear model parameterised with logarithmic link function and negative binomial distribution and with ‘Treatment’ (safinamide 100 mg/day or placebo) and ‘Study indicator’ (016 or SETTLE) as fixed effects. The analysis was performed using the GENMOD Procedure of SAS software version 9.4. The analyses of PDQ-39 domain ‘Bodily discomfort’ and PDQ-39 individual items related to pain were performed using an ANCOVA model with PDQ-39 score changes from baseline as dependent variable, with ‘Treatment’ (safinamide 100 mg/day or placebo) and ‘Study indicator’ (016 or SETTLE) as fixed effects and with baseline values as covariates.
Results
After 6 months of treatment, the proportion of patients not using concomitant pain treatments was significantly greater in the group receiving safinamide 100 mg/day than in the placebo group (respectively, 76.1 % versus 70.0 %; p=0.0305) (see Table 4).

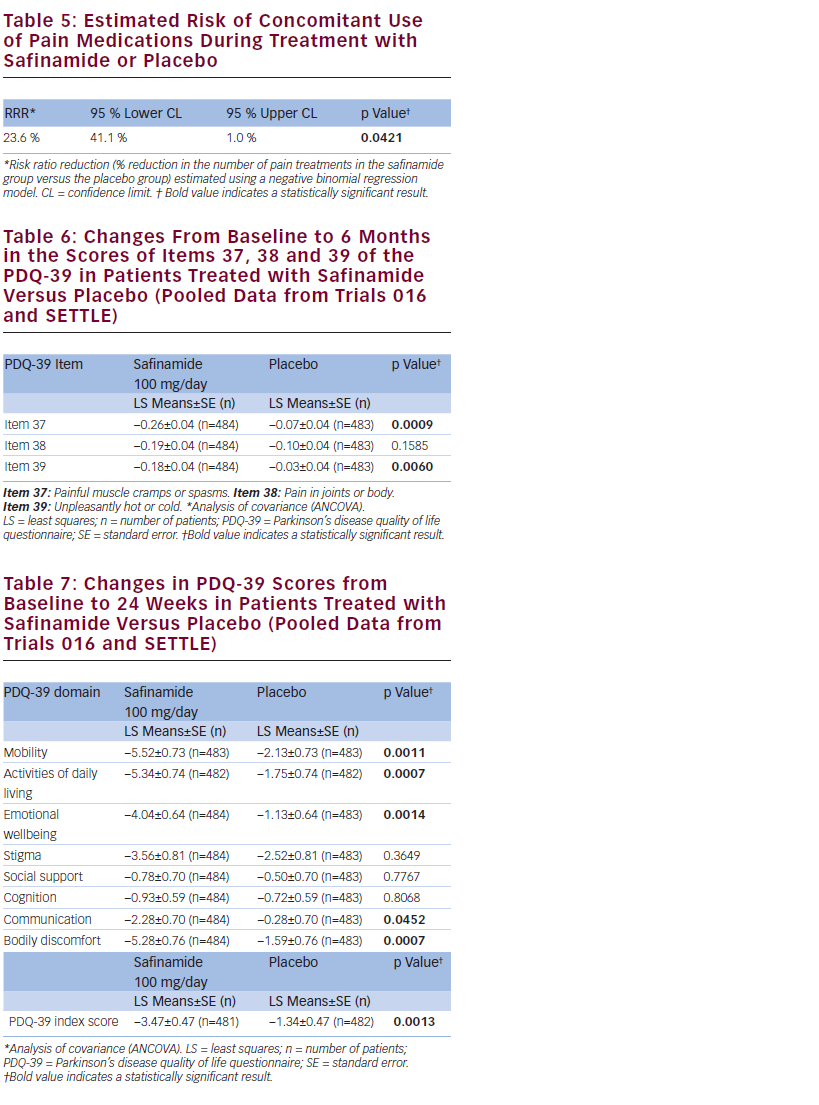
Safinamide 100 mg/day significantly reduced the number of concomitant pain treatments by 23.6 % compared with placebo (p=0.0421) (see Table 5) and was associated with statistically significantly greater improvements in two of the three specific items of the ‘Bodily discomfort’ domain of the PDQ-39, related to neuropathic pain but not with the item related to nociceptive pain (painful cramps or spasm; p=0.0009 and unpleasantly hot or cold; p=0.0060) (see Table 6).
Moreover, safinamide 100 mg/day improved from baseline to 24 weeks significantly more than placebo in five of the eight PDQ-39 domains (Mobility; p=0.0011, Activities of daily living; p=0.0007, Emotional wellbeing; p=0.0014, Communication; p=0.0452, Bodily discomfort; p=0.0007) and in the PDQ-39 index score (p=0.0013) (see Table 7).
Discussion
The findings of this post hoc analyses of the effects of safinamide on motor complications confirmed and extended the results obtained in pivotal studies.
Safinamide add-on was associated with improvements in motor fluctuations without increasing troublesome dyskinesias in PD patients treated with l-dopa alone or in combination with DA-ago or COMT inhibitors, suggesting that safinamide can be considered either as add-on medication in PD patients who are not sufficiently controlled on l-dopa or as an adjunct treatment in patients already taking l-dopa and other dopaminergic medications.10 Another interesting finding was the improvement observed in safinamidetreated patients of bradykinesia, rigidity, tremor and gait. This result is important because the patients were receiving a stable, optimised antiparkinson therapy, so further improvements in the UPDRS scores were unexpected.
The improvement of dyskinesias with the 100 mg dose of safinamide confirmed the previous 018 study results; the significant statistical differences compared with placebo in the subgroup of patients with no changes in l-dopa dose suggest that these improvements were neither due to an adjustment of the l-dopa dose, nor due to reduced dopaminergic stimulation, as demonstrated by the effects of safinamide on motor fluctuations.
The favourable effect of safinamide on dyskinesia in the long term may be explained by its inhibitory action on state- and use-dependent sodium channels and stimulated glutamate release. In a model of l-dopa-induced dyskinesia in MPTP-lesioned macaque monkeys, safinamide treatment caused a dose-dependent reduction in dyskinesia scores, concomitant with an extension in the duration of efficacy against primary parkinsonian symptoms. This antidyskinetic activity was associated with plasma safinamide levels similar to those currently being tested in clinical trials.14
Despite contributing to disease-related discomfort and disability, pain in PD frequently goes overlooked and undertreated in clinical practice.15 Pain intensity has been shown to inversely correlate with the efficacy of dopaminergic medication. Even if l-dopa improves motor symptoms, there is no direct correlation between sensory/ pain changes and motor improvement, suggesting that they do not necessarily share the same mechanisms.16
The loss of dopaminergic neurones causes glutamatergic hyperactivity, the selective inhibition of which could be an effective strategy for the treatment of pain in PD.5 The results observed indicate a favourable effect of safinamide 100 mg/day on pain, which may be explained by its inhibitory action on glutamate release and by the state- and use-dependent blockade of sodium channels and modulation of calcium channels with a significant improvement on the patients’ quality of life.
Safinamide is a unique compound exhibiting a combined dopaminergic and non-dopaminergic mode of action, which modulates altered dopaminergic and glutamatergic neurotransmission.
The results of the above-described post hoc analyses suggest that safinamide improves motor symptoms and controls motor complications (in particular dyskinesia) maintaining its benefits after long-term treatment, and has a positive effect even on pain, one of the most underestimated non-motor symptoms.
It therefore has the potential to become a useful drug in PD management either as add-on medication in patients not optimally controlled by l-dopa alone or as an adjunct treatment in patients treated with l-dopa and a combination of dopaminergic drugs.














