Disease-modifying therapies (DMTs) have brought about substantial improvements to the lives of people with multiple sclerosis (MS).1–3 However, MS remains a chronic disease with a variable course and symptoms, representing a substantial challenge to quality of life.2,3 MS management requires a personalized approach by which healthcare professionals (HCPs) partner with patients.4,5 MS nurses play a central role in MS management and are uniquely positioned to create and maintain these partnerships.4,5 Through individualized communication, MS nurses can help patients understand their condition and treatment while supporting lifestyle choices and encouraging medication adherence.4,5
Purpose and methods
This update summarizes the clinical profile of cladribine tablets in MS and provides a review of practical considerations for nursing care for people with MS who are receiving cladribine tablets, which are topics that have not been explored sufficiently in the literature. Published articles were selected by the authors based on their relevance to the topics included. The author team, consisting of physicians and nurses, agreed on key communication points and developed the example questions (see text boxes) based on their clinical experience to assist HCPs involved in the care of patients with MS.
Management of multiple sclerosis
MS is characterized by immune cells attacking the myelin sheath surrounding nerve cells, resulting in inflammation and destruction of the nerve cells within the central nervous system.2,3 Inflammation and neurodegeneration are present throughout the course of MS, with inflammation causing relapses and the accumulation of new magnetic resonance imaging (MRI) lesions most prominent early in the disease.1–3 As a result, immunomodulating therapies for MS mainly target inflammation and are most effective early in the disease course.2 Current therapies can be divided into continuous (also called maintenance therapies, which exert efficacy while they are administered) and intermittent (sometimes called immune reconstitution therapies).6 Immune reconstitution therapies are given as short courses and can yield longer-lasting efficacy beyond the administration period.6–12 The therapy causes transient reductions in certain types of lymphocytes (Supplemental Digital Content 1 and 2),6–8 which return to within normal limits during the subsequent months.6–8 Clinical data indicate that treatment with cladribine tablets results in durable clinical responses and a favourable risk–benefit ratio.9–11
Cladribine tablets are indicated for adults with highly active relapsing MS in the EU and Australia.7 They are taken orally at home for up to 10 days per year for 2 years only; further cladribine tablets treatment is not recommended in years 3 and 4 (Supplemental Digital Content 1).7 This dosing pattern reflects the long-term effects of a short course of cladribine tablets in people with MS.6 Specialist MS nurses have a key role to play in explaining this dosing and providing reassurance on efficacy and safety.4,5
Nursing considerations for patients prescribed cladribine tablets
| What input can I provide at the start of treatment? An MS nurse meets a patient with highly active relapsing-remitting MS. Six months ago, the patient experienced a sudden and painful loss of vision. More recently, they experienced paresis of the left leg leading to further examinations and an MS diagnosis. The patient has been prescribed cladribine tablets and is concerned about their symptoms and what the new treatment means for them. The MS nurse describes the treatment schedule and provides other practical information needed for starting treatment. Together, the MS nurse and the patient outline a plan for how and when the patient will take the medication. |
Cladribine tablet dosing and pretreatment checks
Eligible patients prescribed cladribine tablets should be informed that, due to the mechanism of action, they may experience lymphopenia.7,8 Lymphocyte counts are required before treatment initiation and re-treatment, as well as at 2 and 6 months after treatment in years 1 and 2 (Supplemental Digital Content 1).7 Counts must be within the normal range before treatment in year 1. In year 2, counts must be ≥800 cells/mm3 before treatment. If necessary, treatment can be delayed for up to 6 months to allow for the recovery of lymphocytes, which is required in some patients.7 Clinical pharmacology data have shown that delaying year 2 treatment this way does not affect efficacy.13 Patients should be informed about possible infection risks resulting from very low lymphocyte counts. If lymphocyte counts drop below 200 cells/mm3, antiherpes prophylaxis should be considered according to local standard practice.7
Screening for pregnancy, active chronic infections (including HIV infection), active malignancy and immunocompromised status is required before treatment in both years 1 and 2.7 Baseline MRI should be performed before treatment in year 1, usually within 3 months (Supplemental Digital Content 1 and 2).7
Cladribine tablet dosing is weight-based, with a total dosage of 3.5 mg/kg body weight over 2 years (one 1.75 mg/kg treatment course per year).7 Each year, there are 4 or 5 days of treatment at the start of month 1 and at the start of month 2. Patients should take cladribine tablets at home with water and swallow the tablets without chewing. The tablets should be stored in their original container, and the patients’ hands should be dry when handling tablets. Hands and surfaces that come into contact with the tablets should be washed after dosing. Missed doses should be taken as soon as possible; however, patients should not take more than one dose on any single day, so a missed dose that is taken the next day should result in an additional day of treatment overall.7
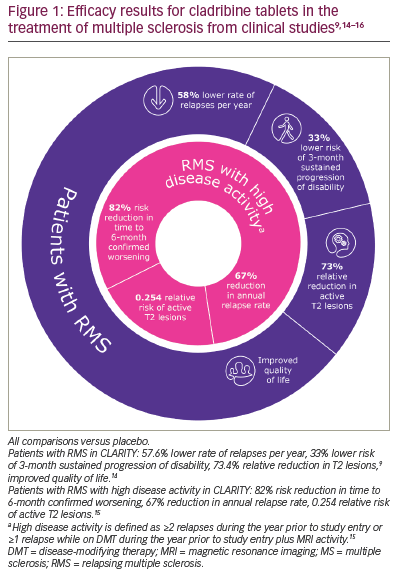
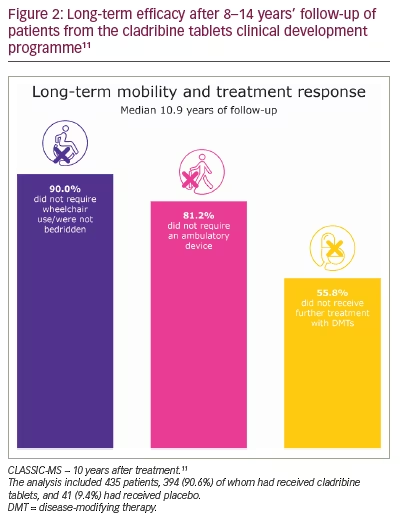
| What information on efficacy can I provide to a patient starting on cladribine tablets? A patient with MS asks what they can expect from treatment. The MS nurse describes the effects observed with cladribine tablets in clinical studies (Figures 1 and 2).9,11,14–16 The MS nurse explains that treatment causes a rapid but transient reduction in certain types of white blood cells, which recover during the following months. This means that the treatment continues working well beyond the very short dosing period. The MS nurse also explains that the patient may not notice a short-term improvement in symptoms while taking cladribine tablets; however, they will be less likely to relapse for several years and to develop a disability, while disease activity detected by MRI is expected to reduce. The MS nurse emphasizes that this treatment may help them control their MS. |
Efficacy of cladribine tablets in multiple sclerosis
Cladribine tablets have been investigated in several MS clinical studies. The CLARITY study (A safety and efficacy study of oral cladribine in subjects with relapsing-remitting multiple sclerosis; ClinicalTrials.gov Identifier: NCT00213135) found that people with MS treated with cladribine tablets had a lower annual relapse rate, greater odds of being relapse free, a longer time to sustained progression of disability, a significant reduction in radiological disease activity on MRI and improved quality of life compared with those on placebo (Figure 1).9,14–16 Improvement in radiological and clinical endpoints was also demonstrated in patients with high disease activity (≥2 relapses during the previous year or ≥1 relapses while on DMT during the previous year plus MRI activity; Figure 1).15 Results from the MAGNIFY-MS study indicated that the treatment effect of cladribine tablets on lesion activity (combined unique active lesions or T1 gadolinium-enhancing lesions) was evident from month 1 (day 30) after treatment initiation (Evaluation of the onset of action in highly active MS; ClinicalTrials.gov identifier: NCT03364036).17
The CLASSIC-MS study is examining the long-term efficacy and effect durability beyond the two annual treatment courses in patients who participated in one of the three largest studies of cladribine tablets (Long-term outcomes and durability of effect following treatment with cladribine tablets for MS; ClinicalTrials.gov identifier: NCT03961204).11 An analysis of 435 patients who had received at least one course of cladribine tablets (90.6%) or placebo (9.4%) found that, at a median of 10.9 years after treatment, 90.0% of patients exposed to cladribine tablets did not require wheelchair use/were not bedridden, 81.2% did not require an ambulatory device and 55.8% did not require further DMT treatment (Figure 2).11
When discussing efficacy, it should be explained that the primary goals of DMT treatment are to reduce clinical disease activity (relapses) and subclinical disease activity (formation of new lesions visible on MRI) to reduce the likelihood of long-term disability.3 Treatment failure is defined by increases in disease activity such as relapses, disability progression and/or new lesions on MRI.3 When using shared decision-making with the patient, it is important to ensure a common understanding of the expected treatment efficacy and the clinical and MRI observations that may prompt a change of treatment (Supplemental Digital Content 3).4,5
As cladribine tablets are administered over 2 years, patients have received only half of the recommended dose after year 1. An analysis of patients who experienced a relapse in the first year of treatment with cladribine tablets found that more than half were subsequently relapse free in the second year,18 indicating that the full treatment effect may not always be evident until year 2.
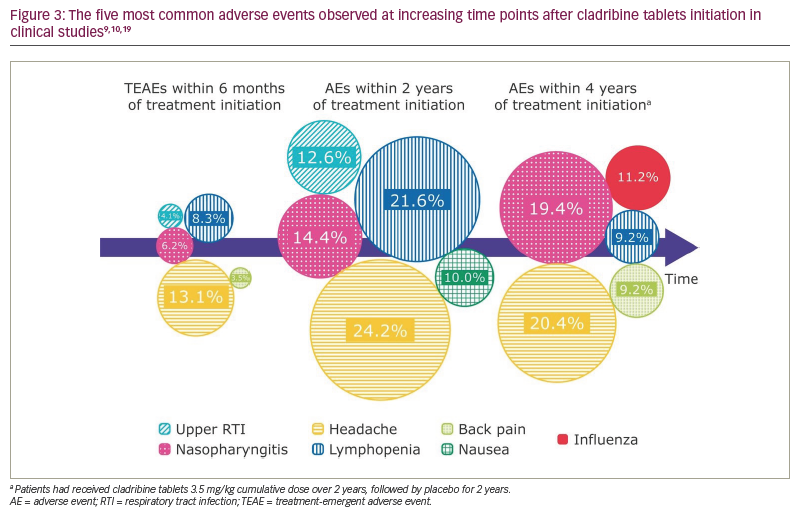
| What safety information can I provide to a patient starting on cladribine tablets? The MS nurse discusses the safety profile of cladribine tablets, including the most common adverse events in clinical studies (Figure 3)9,10,19 and real-world data, and draws on previous personal experience. The patient asks further questions, such as whether they are at greater risk for infections because the treatment affects their immune system, and expresses concern about coronavirus disease 2019 (COVID-19). |
Safety of cladribine tablets in multiple sclerosis
Although each patient will have a different personal experience, the overall safety and tolerability of cladribine tablets are supported by the accounts of thousands of patients with over 16 years of experience (Supplemental Digital Content 3).8–10,12,20–23
Infections are a common concern with many MS treatments due to the effect on lymphocytes. However, the overall infection rates were similar between patients treated with cladribine tablets and those treated with placebo in the CLARITY study, with the exception of herpes zoster, which was reported in 8 out of 430 patients treated with cladribine tablets and in none of the 435 patients who received placebo.9 A long-term follow-up of patients who received cladribine tablets or placebo in clinical studies for up to 8 years showed no differences in the overall incidence of serious infections/infestations (2.5% versus 1.6%, respectively).12 Similarly, the incidence of adverse events of special interest, including viral respiratory infections reported from real-world post-approval sources, were largely consistent with the safety profile for cladribine tablets reported in clinical programmes.23 The majority of opportunistic infections observed were superficial dermal and mucosal fungal infections that resolved with standard treatment; life-threatening opportunistic infections were not observed.23
Rates of infections were higher in patients receiving cladribine tablets during periods of grade 3 or 4 lymphopenia.7,12 Although 20–25% of patients receiving cladribine tablets in clinical studies developed transient grade 3 or 4 lymphopenia, grade 4 lymphopenia was seen in <1% of patients.7 Most patients are expected to recover to either normal lymphocyte counts or grade 1 lymphopenia within 9 months.7
Published pharmacovigilance data to date do not indicate a more serious disease course or more severe outcomes for patients treated with cladribine tablets who acquire COVID-19 compared with the general population or other patients with MS.24 Evidence indicates that patients receiving cladribine tablets can produce antibodies in response to COVID-19 vaccination;25–29 data from a meta-analysis of observational studies indicated that 97.2% of patients receiving cladribine had positive COVID-19 antibody results.28 Furthermore, following the second and third vaccination with a COVID-19 mRNA vaccine, a sustained positive humoral response has been observed in patients with MS receiving cladribine tablets.29 It is still unclear whether patients with MS receiving cladribine tablets have immunoglobulin (Ig) G levels comparable to healthy patients. In one study, IgG levels were significantly lower 1 month after the vaccine dose compared with healthy controls,29 whereas in a different study, IgG levels were comparable between patients treated with cladribine and untreated patients.28 In addition, among a small subset of patients treated with cladribine tablets during phase IV of the MAGNIFY-MS (Evaluation of the onset of action in highly active MS; ClinicalTrials.gov identifier: NCT03364036) and CLOCK-MS (Cladribine tablets: Collaborative study to evaluate impact on central nervous system biomarkers in multiple sclerosis; ClinicalTrials.gov Identifier: NCT03963375) studies who received vaccination for seasonal influenza and varicella zoster virus as standard care, seroprotective antibody titres were either maintained or increased following vaccination.30,31 It should be noted that limited information on the long-term effects of vaccination is available to date, therefore impacting a complete assessment of risk.
In controlled clinical trials, the incidence of fatigue was similar for patients receiving cladribine tablets compared with those on placebo.21 In a real-world setting, individual experience of fatigue with cladribine tablets may vary, and some patients may report a transient increase in fatigue during the weeks that the treatment is given. However, as a common symptom of MS,3,32 it can be difficult to distinguish fatigue due to treatment from MS-related fatigue.
Prior to the approval of cladribine tablets for MS, clinical trials showed a numerically higher number of malignancies in people with MS treated with cladribine tablets compared with those given placebo.7,20 However, the difference was not statistically significant, and the risk of malignancy did not increase over time in patients treated with cladribine tablets (Supplemental Digital Content 4).12,21 Epidemiological analyses found a similar rate of malignancies among patients treated with cladribine tablets as in matched reference populations (i.e. background levels).12
Men and women of reproductive potential must use effective contraception during treatment and for 6 months after each treatment course.7 An examination of the small number of pregnancies that occurred among participants in the clinical trials of the cladribine tablets did not find evidence of increased negative outcomes compared with epidemiological data on women with MS not receiving cladribine tablets or women in the general population.33 If pregnancy does occur within 6 months after the last dose of cladribine tablets, it is recommended that patients be informed of the potential risks to the foetus and referred to a high-risk pregnancy clinic.
Shared decision-making in multiple sclerosis
The goals of achieving greater understanding and establishing shared decision-making between HCPs and patients require effort from both parties.34 At the core of the Four Habits model is effective communication during clinical encounters, including fully eliciting the patients’ concerns/perspectives, conveying empathy, providing a clear rationale for a treatment plan, and showing respect for patients and their concerns. Correctly identifying patient requests, exploring the impact of symptoms on daily lives and involving patients in decision-making allows HCPs to personalize care.34 It is important to remember that information about MS and its treatments may be new to patients, especially those unfamiliar with clinical terminology, and that language and cultural differences may also influence understanding.
Practical nursing considerations for patients with multiple sclerosis prescribed cladribine tablets
Cognitive impairment is seen in MS and may also impair the patient’s ability to understand information and adhere to treatment. Patients may find it helpful to set reminders or alarms for when they need to take their medication. In some cases, it may be necessary to call the patient each day to ensure they take their medication on time, or to make appointments for a more frequent follow-up to ensure adherence. Patients should be encouraged to contact their nurses with questions or concerns whenever necessary. Additional tools are available to support adherence to and satisfaction with treatment (e.g. the adveva® support programme,35 Staying Smart36 and MS in the 21st Century37).
| How can I ensure that my patients feel that they are taking an active part in the management of their multiple sclerosis? Together, the MS nurse and patient have identified a treatment plan that the patient is comfortable with. The patient understands their responsibility in taking their tablets and plans to write down any side effects or changes in disease activity. The nurse agrees to review this with the patient regularly and emphasizes that the patient can always come to them or another HCP with any questions. At the end of the consultation, the patient is still concerned about their MS but is optimistic about treatment. They feel like a partner in their own care and have a plan of action. |
Empowering patients with multiple sclerosis
With increasing treatment choices, partnerships between HCPs and people with MS are crucial for improving quality of care and patient outcomes. MS nurses can explain the effects of cladribine tablets and why they were suggested; this understanding can help to set reasonable expectations and may enhance patient satisfaction. Successful consultations empower patients regarding their own healthcare and encourage shared decision making. Particularly for home-administered treatments such as cladribine tablets, patients should receive practical advice on dosing as well as on monitoring their own health to foster a sense of control over treatment (Supplemental Digital Content 1). Patients should also be aware of relevant safety considerations. It is also important that patients recognize their options for switching treatment depending on their circumstances. MS nurses are in a unique position to provide patients with a full understanding of the implications of treatment and to help them plan for the future.
Although there have been other systematic and exhaustive reviews published for nurses that offer broad overviews of nursing practice in MS,4,5 the approach taken for this article was the development of a concise overview of the key features of cladribine tablets to help MS nurses treat patients. This update addresses key questions that have been raised during interactions with patients receiving cladribine tablets. It is hoped that the practical aspects discussed here will help colleagues to better interact with and manage patients.
Conclusions
MS nurses play an important role in supporting people with MS who are prescribed cladribine tablets, from reassurance at treatment initiation to support with medication adherence and setting clear shared goals. Effective communication at consultations can be invaluable for patients, helping to inform them, increase comfort around treatment and increase satisfaction with care.