Multiple sclerosis (MS) is a chronic, immune-mediated disorder of the central nervous system characterized by inflammation, demyelination and neurodegeneration. Natalizumab is a widely used anti-α4 integrin inhibitor for treating highly active MS. In the pivotal trials of natalizumab for MS, the SENTINEL and AFFIRM trials, natalizumab was established to reduce the rate of clinical relapses and slow the progression of disability.1,2 Clinical and radiological relapses were observed at rates of 5% of 589 (SENTINEL) and 6% of 627 patients (AFFIRM). There are, however, case reports of severe disease activity paradoxically occurring after the initiation of natalizumab treatment. To date, only five such cases of the early emergence of tumefactive demyelinating lesions while taking natalizumab have been reported in the literature.3–7 In two of these cases, anti-natalizumab antibodies were detected.5,6 This case report aims to contribute to the understanding of this rare phenomenon, providing insights into a case where a patient experienced an increase in disease activity after the second dose of natalizumab.
Case description
A 37-year-old female patient was diagnosed with MS after presenting with left optic neuritis and sensory disturbances in the lower limbs. Magnetic resonance imaging (MRI) of her brain was normal, but an enhancing lesion was observed at T5 on spinal imaging. Neuromyelitis optica spectrum disorder (NMOSD) was considered, but cerebrospinal fluid (CSF) and blood assays for anti-aquaporin-4 (AQP4) immunoglobulin (Ig)G antibodies and antimyelin oligodendrocyte glycoprotein antibodies were negative. The single-level spinal lesion did not meet the diagnostic criteria for seronegative NMOSD. The presence of unmatched oligoclonal bands in the patient’s CSF confirmed the diagnosis of MS.
Compliance with ethics
Case reports not subject to ethics approval by The Research Ethics Committee of St. Vincent’s Healthcare Group. Written informed consent was received from the patient involved in this case study for the publication of the case report and the images.
Initially, the patient was treated with glatiramer acetate, which was effective at first. After a relapse of similar symptoms, the patient switched to dimethyl fumarate; however, further disease progression led to a switch to natalizumab (without a washout period) 4 months later. An MRI of the patient’s brain one week prior to starting natalizumab showed non-specific T2/fluid attenuation inversion recovery hyperintensities, but no abnormal enhancement (see Figure 1A). Before starting treatment with natalizumab, the patient’s Expanded Disability Status Scale (EDSS) score was 2.0.
Figure 1: Magnetic resonance imaging of the case report participant
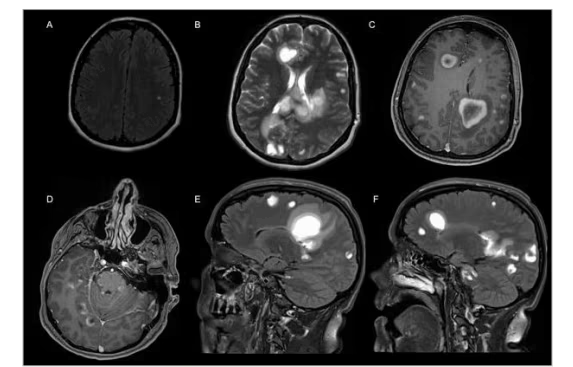
A: Axial fluid attenuation inversion recovery pre-natalizumab commencement shows a number of hyperintense lesions in both hemispheres; this is new from her previous scan, but none enhanced post gadolinium. B: Axial T2 sequence shows hyperintense tumefactive type lesions in the periventricular white matter, the corpus callosum and subcortical white matter. C, D: Axial T1-weighted images post contrast show enhancing lesions in both cerebral hemispheres, the cervicomedullary junction, the cerebellar peduncles, cerebellar hemispheres and around the brainstem. Most of the lesions demonstrated enhancing rims which were incomplete or C-shaped, broken facing towards the cortex. E, F: Sagittal T2-weighted images show extensive elongated flame-shaped hyperintense lesions.
After receiving two doses of natalizumab, the patient experienced significant clinical deterioration and presented with tetraparesis, impaired cognition, bed-boundness, incontinence and poor responsiveness. An MRI of the patient’s brain showed a dramatic deterioration hitherto unseen in her clinical course (see Figure 1B–F). Spinal imaging was not repeated at the time. Full blood count and blood chemistries were normal. The workup for infectious disease (human immunodeficiency virus, hepatitis B and C, toxoplasmosis, cytomegalovirus, serology for Treponema and Borrelia) was unremarkable, as was a vasculitic disease workup (antineutrophil cytoplasmic antibody, antinuclear antibody, extractable nuclear antigens, rheumatoid factor, serum paraproteins, complement, lupus anticoagulant, anticardiolipin antibodies, angiotensin-converting enzyme, C reactive protein and erythrocyte sedimentation rate). The CSF showed no pleocytosis but a raised protein level of 1435 mg/dl (15–60 mg/dl). The polymerase chain reaction (PCR) for John Cunningham (JC) virus in CSF was negative. Repeat CSF and blood assays for anti-AQP4 IgG antibodies and antimyelin oligodendrocyte glycoprotein antibodies were again negative.
Due to concerns about progressive multifocal leukoencephalopathy or lymphoma, and because brainstem leptomeningeal enhancement detected on the MRI was considered atypical for MS (see Figure 1D), a right frontal brain biopsy was performed. The biopsy confirmed demyelination, and the patient was treated with intravenous methylprednisolone (IVMP), intravenous Ig and plasma exchange for 5 days. The patient improved both clinically and radiologically but had persistent, significant, right-sided hemiparesis. The patient provided written consent for the publication of a case description and images. Information on the subsequent clinical course and later follow-up is not available, which is a limitation of this case report.
Discussion
Our case is one of significant clinical worsening associated with the radiological development of tumefactive demyelinating lesions, apparently in response to natalizumab initiation, in the absence of anti-natalizumab antibodies, with significant patient morbidity.
Tumefactive demyelinating lesions (TDLs) are central nervous system manifestations of MS, myelin oligodendrocyte glycoprotein-associated disease, and AQP4 antibody-positive NMOSD. They can also occur as an idiopathic monophasic disease. They are characteristically >2 cm in size, locally aggressive and space-occupying. As the name suggests, they can mimic tumours in their clinical, radiological and pathological features.8,9
Our case has similarities with several other isolated case reports of paradoxical TDL development after natalizumab initiation in timeline, clinical findings, investigation results and outcome. These cases are reviewed and compared below.
Debs et al. reported a case of extensive infiltrating TDLs developing in a patient after 10 doses of natalizumab in a patient previously treated with interferon beta.6 The anti-natalizumab antibody was positive, but no titre was available. After treatment with ten consecutive pulses of IVMP, the patient improved clinically. Interferon beta-1a was reinitiated after 6 months, and no relapses occurred in the following year. The EDSS was 7.0 at the last review, having been 3.5 prior to natalizumab commencement.
In another case with anti-natalizumab antibodies, Svenningsson et al. reported a patient who developed chills and fever after the fourth and fifth doses of natalizumab and required hospitalization after the sixth dose due to ataxia and reduced mental status.5 The patient’s clinical status deteriorated further, with the patient exhibiting tetraplegia and depressed consciousness, and the MRI showed an increase in hyperintense T2 lesions and multifocal areas of gadolinium enhancement compared with prior studies. The patient had a high titre of anti-natalizumab antibodies (335 mg/L). She was treated with plasma exchange, and the authors were considering autologous stem cell transplantation before the patient died from the illness.
In these two cases of severe disease activity associated with anti-natalizumab antibodies, the authors posit that disease activity might be related to the antibody working to counteract drug efficacy, and leading to a rebound-like effect. The prevalence of anti-natalizumab antibodies in treated populations was reported to be about 8% in a study of 134 patients.10 The study also showed that antibodies correlate inversely with drug levels and can be detected before the fourth month of treatment.
Debs et al. also suggest that integrin-mediated signalling plays a role in oligodendrocyte maturation and survival and that anti-natalizumab antibodies may interfere with the oligodendroglial integrin-signalling cascade.11 They propose that this phenomenon could be studied using in vitro models of myelination.
Extensive disease relapse with the emergence of TDLs has also been reported in the absence of natalizumab antibodies, as in our case. Berger described a patient who was treated with interferon beta-1a for 4 years prior to switching to natalizumab.3 After four doses of natalizumab, the patient’s clinical status deteriorated, and they exhibited symptoms of ataxia, dysmetria and bilateral limb sensory disturbance. The MRI showed extensive new T2 and fluid attenuation inversion recovery hyperintensities, as well as enhancing C-shaped lesions, to an extent not seen in any of the patient’s previous imaging studies. In another natalizumab antibody-negative case, a patient who had completed four doses of natalizumab developed a large right temporoparietal subcortical lesion which was concerning for multifocal leukoencephalopathy.4 The CSF showed a lymphocytic pleocytosis with 14 and 28 white blood cells/mm3, and CSF JC virus PCR was negative.
Lastly, Moghadasi et al. added to the body of related case reports with one of visual blurring and tumefactive lesions on MRI that occurred after five doses of natalizumab in a patient previously treated with glatiramer acetate and azathioprine.7 Blood tests for anti-AQP4 IgG antibodies, antimyelin oligodendrocyte glycoprotein antibodies and JC virus were negative. A lumbar puncture was not performed due to concern about herniation. Anti-natalizumab antibodies could not be measured. Symptoms, radiological findings and EDSS improved after IVMP.
The similarities between our case and the five cases described above are compared and contrasted in Table 1.
Table 1: Comparing common features in cases of paradoxical tumefactive worsening of multiple sclerosis after natalizumab initiation
| Case | Time since MS diagnosis | Pre-natalizumab treatment(s) | Pre-natalizumab washout | Number of natalizumab doses | Anti-natalizumab antibodies | Ultimate clinical outcome |
| 37 yo female (our case) | 1 year | Glatiramer acetate, then dimethyl fumarate | None | 2 | Negative | Improved after IVMP, IVIG and PE, but persistent significant right hemiparesis |
| 31 yo female3 | 7 years | Interferon beta-1b, Interferon beta-1a | 1 month | 4 | Negative | Radiological improvement (clinical status not documented) after 5 days of IVMP, with glatiramer acetate and pulsed IVMP thereafter |
| 27 yo male4 | 2 years | Interferon beta-1a and weekly pulsed IVMP | Not reported | 4 | Negative | Radiological improvement and mild residual symptoms after PE, glatiramer acetate and monthly pulsed IVMP |
| 32 yo female5 | 6 years | Interferon beta-1a | Not reported | 6 | Positive | Death |
| 40 yo female6 | 14 years | Interferon beta | Not reported | 10 | Positive | Slow improvement after pulsed IVMP and interferon beta. EDSS 7.0 (3.5 pre-natalizumab) |
| 33 yo female7 | Not reported | Glatiramer acetate, azathioprine | Not reported | 5 | Not tested | Radiological, symptom and EDSS stabilization with IVMP |
EDSS = Expanded Disability Status Scale; IVIG = intravenous immunoglobulin; IVMP = intravenous methylprednisolone; MS = multiple sclerosis; PE = plasma exchange; yo = year old.
Additionally, the case of a patient who worsened significantly after a single dose of natalizumab after a brief treatment hiatus was reported.12 The authors postulate that natalizumab may unfavourably influence the immune system in a pro-inflammatory way if initiated during an acute relapse, possibly supporting pre-existent brain inflammation by reducing anti-inflammatory cells and thus fueling B-cell activity and subsequent inflammation.12,13 In this case, however, the patient had extensive enhancing lesions (>40) when natalizumab was administered, in contrast to our patient.
TDLs associated with natalizumab use can occur in patients with and without anti-natalizumab antibodies (as seen in Table 1). The underlying mechanisms are unclear and may be influenced by various factors, including the presence or absence of anti-natalizumab antibodies, the timing of natalizumab administration and the level of pre-existing disease activity.
Importantly, there is observational evidence that natalizumab is ineffective for NMOSD and sometimes may even exacerbate the disease;14 consequently, NMOSD should be confidently ruled out if natalizumab therapy is being considered for a MS diagnosis. Some cases of supposedly unexplained MS disease progression on natalizumab could, in fact, be misdiagnosed NMOSD.
In our case, it is possible that natalizumab directly triggered an inflammatory response by altering the balance of immune cells in the CNS, as described above, that it failed to suppress aggressive disease that would have occurred independent of natalizumab administration, or that the flare of disease activity was a rebound effect, as the effects of dimethyl fumarate waned before natalizumab had the opportunity to reach its full therapeutic effect.
Extensive disease activity or paradoxical worsening on natalizumab is unusual. Our case adds to the sparse literature documenting these cases. Clinical or radiological worsening during natalizumab treatment should prompt testing for anti-natalizumab antibodies; however, these may be negative, as in our report. In these cases, natalizumab treatment should be discontinued and thereafter avoided until more is known about the phenomenon. The possibility of NMOSD should also be considered where MS fails to respond or worsens with natalizumab. While theories exist to explain why MS worsening may occur in the presence of anti-natalizumab antibodies, explanations for the antibody-negative cases are less established. Further identification and analysis of such cases are necessary to better characterize this phenomenon, its mechanisms, risk factors and how it might be mitigated.