**The expert faculty involved in this supplement were interviewed after the Bial-sponsored satellite symposium held at the 2019 International Congress of Parkinson’s Disease and Movement Disorders, Nice, France. Their interviews, relating to the highlights of the symposium, have been included within the HTML version of supplement below, and were filmed with support from Bial.**
Introduction
Olivier Rascol
Clinical Investigation Center, Toulouse University Hospital, INSERM & University of Toulouse, Toulouse, France
During the course of this symposium report we will review the clinical spectrum of wearing-off symptoms, including both motor and non-motor features; the challenges we still face in many patients despite an increased understanding of wearing-off; and our current understanding of the ON–OFF phenomena time course and how this has evolved beyond the classical view of Parkinson’s disease (PD) staging. _EPUB-web-resources/image/1.png)
The spectrum of ‘OFF’ in Parkinson’s disease
Hubert Fernandez
Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH, USA
Wearing-off refers to the recurrence of motor (and non-motor) symptoms in patients receiving anti-parkinsonian medication, typically preceding scheduled doses. During wearing-off periods, motor symptoms are usually characteristic of PD (e.g., recurrent tremor, walking/balance impairment, slowness and/or stiffness of movement), while non-motor symptoms (NMS) are more varied and comprise both autonomic and neuropsychiatric symptoms, including pain, panic attacks and bladder problems (Figure 1).1 Wearing-off is a common occurrence, with >50% of patients with PD experiencing fluctuations in their response to levodopa after 5–10 years of treatment.2,3 Indeed, a real-world survey study in over 3,000 patients with PD conducted by the Michael J Fox Foundation showed that >90% of patients experienced at least one wearing-off episode, and 70% experienced at least two episodes in a typical day.4 Further, wearing-off has been shown to be significantly bothersome to the patient (particularly motor symptom fluctuations in patients with advanced PD),5 have a pronounced negative effect on the patient’s motor function, and result in impaired health-related quality of life (HRQoL).3,6 In a study of 143 patients with PD, wearing-off was shown to significantly worsen patient quality of life as assessed using the Parkinson’s Disease Questionnaire (39-item version; PDQ-39), with mobility, activities of daily living, and communication most strongly affected.7 In particular, nocturnal akinesia resulted in a deterioration of all dimensions of the PDQ-39.7 Wearing-off also has a substantial economic impact, with annual medical costs increasing with the proportion of time spent in the ‘OFF’ state.8 Interestingly, only a small percentage of the total costs are direct medical costs, with indirect costs and direct non-medical costs making up the large proportion of the total economic burden.8
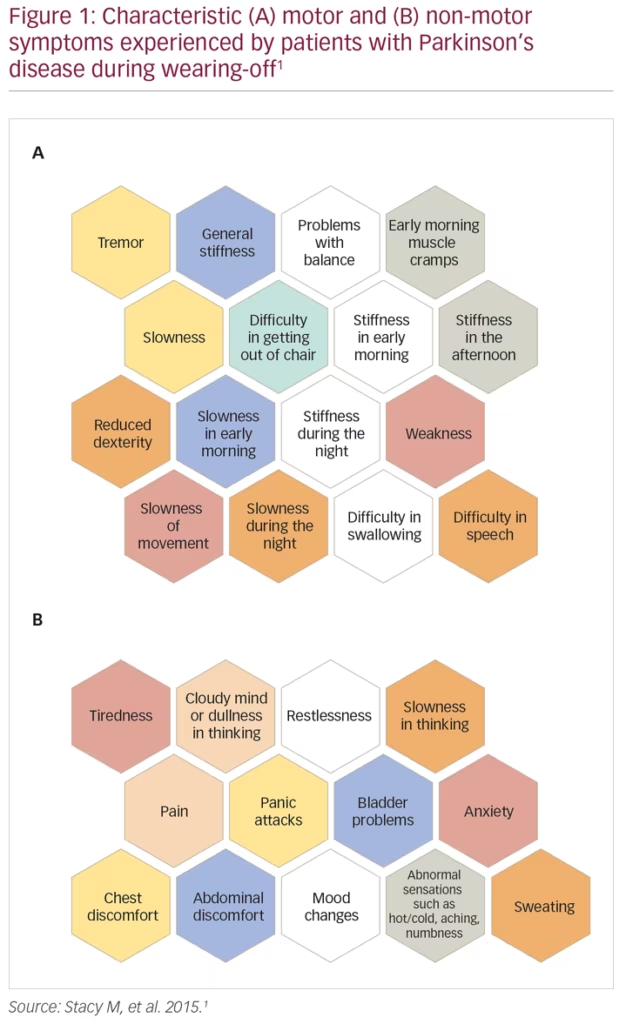
Despite the common nature of wearing-off and the substantial burden it exerts on both the patient and the healthcare system, it remains an under-recognised problem. Potential reasons for this include limited evaluation time for physicians, miscommunication between patients and caregivers, particularly with regard to the reporting of NMS such as cognitive dysfunction (or ‘brain fog’) and urinary problems, and the lack of an established definition for wearing-off. Current definitions put a heavy emphasis on motor symptoms, potentially missing ‘transition states’ between ON and OFF periods. This lack of an established definition is reflected in the results of a study by Stacy et al., where clinicians were shown to identify wearing-off in only 29.4% of cases, fewer than specific questionnaires such as the Unified Parkinson’s Disease Rating Scale (UPDRS) Part IV, Question 36 (43.9%) or the wearing-off patient questionnaire (WOQ-19; 57.1%).1 However, even the gold-standard questionnaires are reliant on patient recall and only usually assess symptoms across a single day. It is hoped that with the advent of new mobile technologies (e.g., smartphones, cloud computing) it will be possible to assess patients more closely and for longer periods of time.9
In summary, the proposed definition of ‘OFF’ is a temporary change in the clinical state of a patient with PD, which is perceived as negative when compared with the beneficial effects of PD therapy.10 While it can be broadly characterised by specific motor symptoms and NMS, the presentation of ‘OFF’ is unique for each patient. As such, the ‘OFF’ spectrum incorporates a variety of disease states and terms, including (but not limited to) wearing-off, ON–OFF phenomena, early morning akinesia, delayed ON periods, dose failures and OFF-period dystonia.10 As there is still no formal definition of wearing-off, there is a clear need to increase awareness of the phenomenon with appropriate medical education and use of specific tools such as the WOQ-19. _EPUB-web-resources/image/1.png)
The time course of non-motor complications
Per Odin
Division of Neurology, Lund University, Lund, Sweden
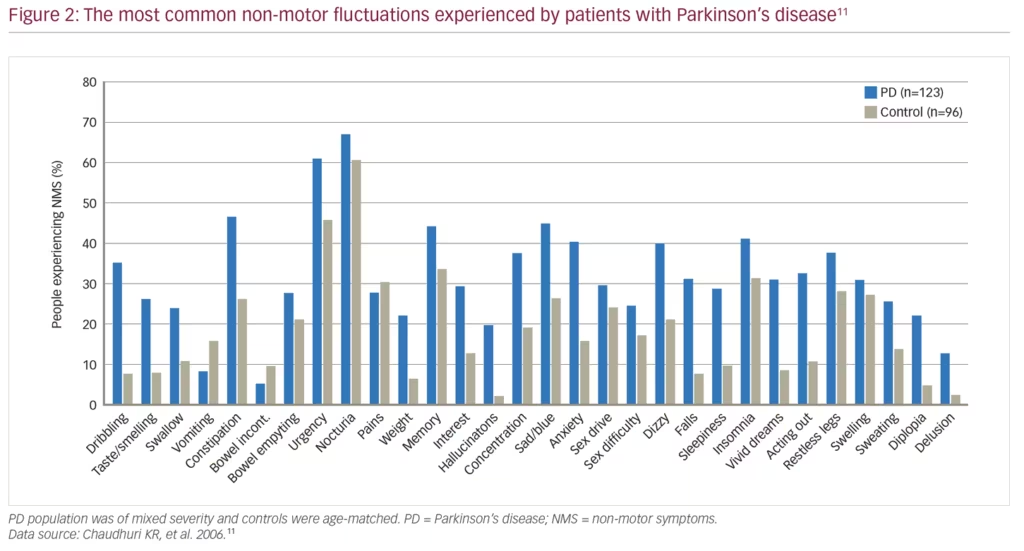
There has been a growing awareness over the last few decades of the importance of NMS in PD. NMS are common in PD, with the NMSQuest study finding that most individual symptoms, ranging from urinary to psychiatric symptoms, were more common in patients with PD (n=123) than in age-matched healthy controls (n=96) (Figure 2).11 NMS are present even in newly diagnosed patients, with the longitudinal, multicentre PRIAMO study of 1,072 patients with PD demonstrating that psychiatric (61.1%), pain (50.9%) and gastrointestinal (45.5%) symptoms are frequently present even in patients at Hoehn and Yahr disease stage 1.12 Furthermore, a range of NMS are more common in patients with PD compared with matched controls in the 5 years before diagnosis including constipation, fatigue, dizziness, hypotension, erectile dysfunction, urinary dysfunction, depression, anxiety, insomnia and memory problems; by contrast motor symptoms are not evident until later in the disease course.13 As PD progresses, the incidence of NMS continues to increase, and eventually leads to the onset of symptoms such as dementia, cognitive dysfunction, hallucinations, incontinence, sexual dysfunction and orthostatic hypotension.14
The progression of individual NMS can vary considerably. The PRIAMO study found that over a 24-month period (n=707), skin (+19% incidence), attention/memory (+13%), gastrointestinal (+12%) and sleep symptoms (+11%) were the most progressive NMS, whereas cardiovascular (-22% incidence), psychiatric (-18%) and respiratory (-13%) were the least progressive symptoms.15 One observational study following 61 patients with untreated PD for 4 years found that the greatest increases in the incidence of individual NMS between 2 and 4 years post-diagnosis were for nausea/vomiting, sex drive, hallucinations, swallowing difficulties, daytime sleepiness, dizziness and nocturia, whereas, in contrast, incidence of delusions, sex difficulties and weight change were affected the least, between 2 and 4 years post-diagnosis.16
NMS have a large impact on patients with PD throughout the disease course. A questionnaire study of 265 patients with early and late stage PD (<6 and ≥6 years of disease, respectively) investigated the most troublesome symptoms that patients experienced in the last 6 months.5 In early-stage PD (n=92), the majority of the top 15 most troublesome, score-weighted symptoms experienced by patients were non-motor in nature; after the motor symptoms (slowness, tremor and stiffness), pain (25.0%), loss of smell/taste (16.3%), mood (15.2%), handwriting (12.0%) and bowel problems (10.9%) were ranked the highest. In late-stage PD, after ‘fluctuating response to medication’, NMS represent the next eight most highly ranked troublesome symptoms, including mood symptoms (28.3%), drooling (21.4%), sleep symptoms (23.1%), tremor (17.3%), pain (16.2%), bowel problems (14.5%), urinary problems (12.1%), falls (10.4%) and symptoms relating to appetite/weight (11.6%).5 The impact of NMS in PD is present even in the earliest stage of the disease, with one cross-sectional, multicentre study finding that 21% and 15% of treated patients (n=170) had severe or very severe NMS in early PD, and even 22% and 19% of the earliest stage patients (naïve to treatment [n=64]) had severe and very severe NMS.17
Several studies have demonstrated the association between NMS and HRQoL as measured by the PDQ-8, PDQ-39 and EuroQol-5 dimensions.18,19 On an individual symptom level, despite the frequency of symptoms such as nocturia (68.4%), fatigue (65.9%) and dribbling saliva (56.7%) in PD, HRQoL has been reported to be most significantly impacted by sleep/fatigue and mood/apathy.20 These results are consistent with the PRIAMO study, which found that the impact of individual NMS on HRQoL was not related to NMS incidence. The study found that cardiovascular NMS has the greatest negative impact on HRQoL, followed by apathy, urinary symptoms, psychiatric symptoms and fatigue.15
As with motor symptoms, patients with PD can also experience non-motor fluctuations (NMF).21 The first described NMF was in depression, although fatigue is frequently reported to be the symptom with the most common fluctuations, and sensory and pain fluctuations the most disabling.21,22 The nature of NMF is heterogeneous and complex, with some NMS such as anxiety, depression and fatigue fluctuating in parallel with motor fluctuations, while other NMS do not follow this pattern.21,23
The detection and treatment of NMS and NMF present several challenges in the management of PD. Clinically, there are no widely accepted rating instruments for NMF, although the WOQ-19 and modified NMS scale have both been demonstrated to successfully identify NMF, and the Movement Disorder Society-sponsored Non-motor Rating Scale (MDS-NMS) is currently under investigation.21,22,24 The initial approach to treating NMF is consistent with the treatment of motor fluctuations, namely achieving continuous dopaminergic stimulation with the addition of catechol-O-methyltransferase (COMT) inhibitors where required.21 However, there is little current evidence for the efficacy of specific therapies, although several NMS are known to be responsive to dopamine therapy.25 These include gastrointestinal symptoms such as constipation and unsatisfactory voiding; autonomic symptoms including bladder urgency, nocturia and erectile impotence; neuropsychiatric symptoms including depression, apathy, anhedonia and panic attacks; sleep symptoms including restless leg syndrome, periodic limb movement and rapid eyeball movement behavioural disorder; sensory symptoms including central pain and pain related to fluctuations, as well as to fatigue.25 Most recently, advanced PD therapies including deep brain stimulation of the subthalamic nucleus, intrajejunal levodopa infusions and apomorphine infusions have demonstrated efficacy in reducing specific NMS; the former two treatments especially reduce sleep/fatigue, and all three reduce mood/cognition symptoms.26
In summary, NMS are frequent in patients with PD, can occur early in the disease, even preceding motor symptoms, and most individual symptoms are progressive. As such, the earlier a diagnosis of wearing-off can be made, the earlier treatment can be initiated to alleviate the patient’s symptoms. NMS, particularly depression and sleep, have an important impact on HRQoL. Treatment options are to provide more continuous dopaminergic stimulation, and to target individual NMS. _EPUB-web-resources/image/1.png)
The time course of motor complications – what are we missing?
Joaquim Ferreira
Faculdade de Medicina, Universidade de Lisboa and CNS – Campus Neurológico Sénior, Torres Vedras, Portugal
The classical view of PD staging is that patients begin to develop motor fluctuations (MF) in the form of early morning akinesia and wearing-off, followed by ON–OFF fluctuations, and that dyskinesias develop from non-troublesome dyskinesia into troublesome dyskinesia in the advanced stages of PD. However, a range of studies have challenged the onset timing, predictors, incidence in advance disease and response to therapy of MF and dyskinesias.
Both MF and dyskinesia can be present in the early stages of the disease, with one literature analysis demonstrating that 31% of patients have these symptoms after 2.5–3.5 years, 41% after 4–6 years and 70% after ≥9 years of disease.27 Additionally, although wearing-off has often been a focus of PD management, the time to ‘ON’ (i.e., the time during transitions from OFF to ON states) is now recognised as a major component of total daily OFF time, comprising an estimated 68%.28
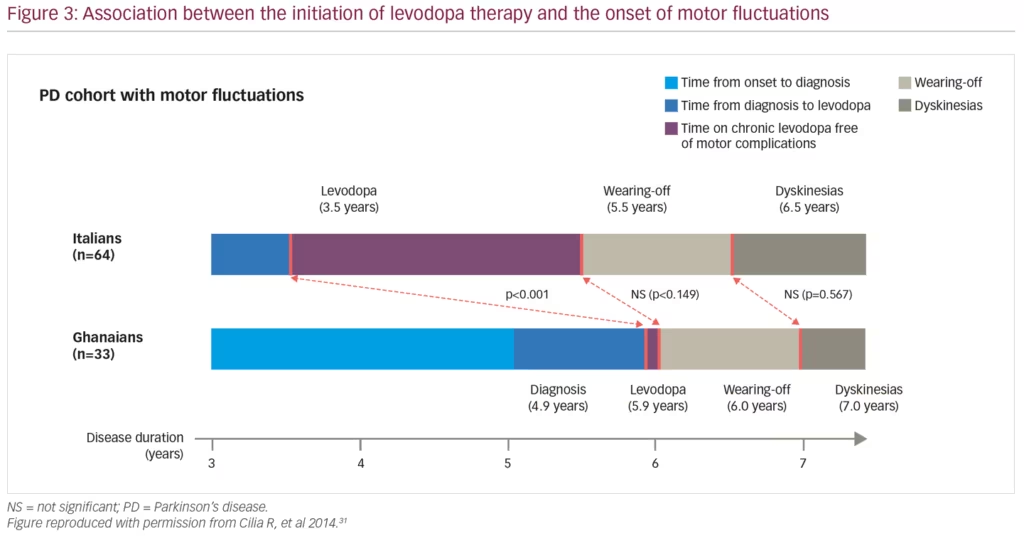
MF and dyskinesia can begin earlier than previously thought following the initiation of dopamine therapy. One randomised, double-blind study of 361 patients with Hoehn & Yahr scale stage 3 (or less) PD demonstrated that after 9 months of levodopa therapy 16.5% and 29.7% of patients on the highest levodopa dose of 600 mg/day had developed dyskinesia and wearing-off, respectively.29 As this levodopa dose also resulted in the greatest reduction in UPDRS score, the study highlights the need to balance efficacy with potential adverse events. Another important point is that wearing-off is not limited to levodopa, with a randomised controlled study demonstrating that 7.3% and 3.3% of patients receiving the dopamine agonist, pramipexole, compared with 14.6% and 4.7% receiving levodopa, experienced wearing-off and dyskinesias, respectively.30 A 4-year, multicentre cohort study found that disease duration and levodopa daily dose were associated with motor complications, but not the duration of levodopa therapy (Figure 3).31 Furthermore, a post-hoc analysis of 745 patients from the randomised, double-blind STRIDE-PD (Stalevo Reduction in Dyskinesia Evaluation in Parkinson’s Disease) study found that a range of factors were predictive of dyskinesias including young age at PD onset, higher levodopa dose, low body weight, north American geographic region, female gender and more severe UPDRS Part II score.32 A second, more recent prospective study of 740 patients with PD also found that low mood/anxiety were associated with the onset of MF and dyskinesias, suggesting a link between motor and NMS.33
Finally, MF have traditionally been thought to proceed dyskinesia. However, an analysis of 189 patients from the CALM-PD (Comparison of the Agonist Pramipexole With levodopa on Motor Complications of Parkinson’s Disease) study demonstrated that 12.2% of patients on levodopa or pramipexole developed dyskinesia without MF and 17.5% developed dyskinesia before MF.34 Further, in the pragmatic, open-label PD MED study (long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease), after 7 years of levodopa or levodopa sparing treatment, 33–35% of patients had dyskinesia and 53–56% had MF.35 The LARGO (Lasting effect in Adjunct therapy with Rasagiline Given Once daily) and BIPARK (Efficacy and Safety of BIA 9-1067 in Idiopathic Parkinson’s Disease Patients With “Wearing-off” Phenomenon) studies highlighted that with a disease duration of 7.0–9.2 years, patients have 5–7 hours per day of OFF-time.36,37 COMT inhibitors are recommended by the MDS as a clinically useful intervention for MF in PD,38 with opicapone reducing OFF time by approximately 1 hour and entacapone by 40 minutes compared with placebo.36,39
The occurrence of MF and dyskinesias may not be progressive throughout the disease course of PD. A cross-sectional study of 50 patients with Hoehn & Yahr stage 4 and 5 PD (mean age 74 years) found that although 78% and 62% of patients had wearing-off and dyskinesias after a mean of 18 years disease duration, only 4% and 8% had ON–OFF phenomena and disabling dyskinesias, respectively, and 38% had no dyskinesia.40 Similarly, two other studies (n=61 and n=52, respectively) including patients with PD duration of approximately 15 years also found ON–OFF phenomena and disabling dyskinesias present in only 35–69% and 10–12% of patients, respectively.41,42 It should also be noted that the late stages of PD are still responsive to levodopa, as demonstrated in a study of 20 patients with late-stage PD (mean age 79 years and mean disease duration 14 years), where a significant improvement in MDS-UPDRS Part III total score time with levodopa has been demonstrated.43
In summary, our understanding of PD has evolved beyond the classical view that MF and dyskinesias develop only later in the disease following levodopa therapy. MF and dyskinesia may develop early in the disease, time to ON is a major component of daily OFF time, ON–OFF phenomena and dyskinesias may improve at later stages of the disease and patients can remain responsive to levodopa even in the last stages of the disease. However, current adjunctive treatment options, such as COMT inhibitors can help minimise the impact of wearing-off. _EPUB-web-resources/image/1.png)














