Deep brain stimulation (DBS) is an effective treatment that significantly reduces disabling levodopa-induced motor complications (i.e. dyskinesia and motor fluctuations) and tremor in individuals with Parkinson’s disease (PD).1,2 During DBS, electrical stimulation is delivered through stereotactically implanted electrodes into one of two primary targets considered for PD: the subthalamic nucleus or the globus pallidus internus. The therapy reduces OFF time (i.e. periods of poor motor function) by an average of 69%, while controlling dyskinesia.3,4 Long-term clinical outcomes include sustained improvements in motor complications and a stable reduction in medications by an average of 42–50%.5,6 With respect to the non-motor symptomatology of PD, clinical studies have also demonstrated beneficial effects of DBS on sleep, cardiovascular symptoms, neuropsychiatric disorders (i.e. depression, anxiety) and urinary dysfunction.7,8
Currently, high-frequency stimulation drives therapeutic benefit, but requires time-consuming programming sessions, often relying on a trained clinician with extensive experience and established programming approaches9 to achieve optimal clinical outcomes through a trial-and-error approach. The benefit of stimulation is predicated upon the centroid of the stimulated field being present in the motor area of the target, which, in the case of the subthalamic nucleus – the most common target in treating PD – lies in the dorsolateral region of the structure.10,11 Spread of stimulation beyond the boundaries of the intended target region can cause side effects such as dysarthria, paraesthesia, oculomotor disturbances and muscle contractions.
Customizing the size, shape and intensity of the stimulation field underlies the therapeutic agency of DBS. However, conventional leads typically generate radial fields with limitations on spatial modification. Directional (d)DBS uses leads with segmented electrodes that are capable of steering current in specific directions and modifying the stimulation field. This technique offers the opportunity to improve motor outcomes in patients with PD by directing stimulation to more discrete regions of the implanted target, while minimizing the risk of spread into adjacent white matter tracts. Furthermore, this advance in the spatial control of stimulation is likely to reduce the time-consuming programming sessions needed to achieve clinical optimization. In this article, we review the use of dDBS in treating patients with PD, including its scientific rationale and integration in clinical care.
Scientific rationale for directional deep brain stimulation
Volume of tissue activation and anatomical targets
The goal of DBS stimulation is to maximize clinical benefit while diminishing the stimulation-evoked side effects that arise from the spread of the volume of tissue activated (VTA) beyond the boundaries of the target. While the physical parameters of leads vary across devices,12 they typically have four cylindrical contacts variably interspaced to span 7.5–15 mm from the tip of the electrode.13 Radially shaped VTA fields aligned along the horizontal axis are created by activating one or more cathodic contacts (i.e. monopolar, double monopolar). Smaller VTA fields may be produced by activating contacts as a combination of cathodes and anodes (i.e. bipolar stimulation);14 however, this does not change the overall spherical configuration of the field, but rather modifies the radial diameter of the VTA.
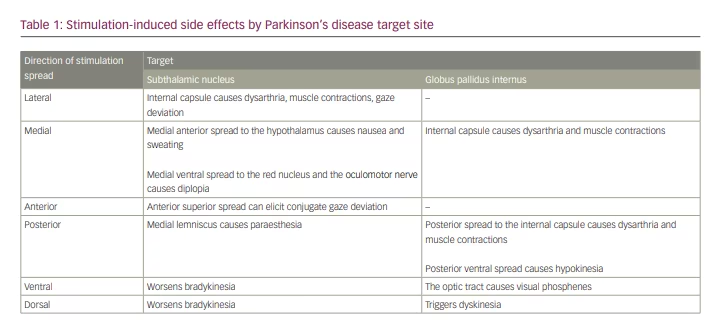
The subthalamic nucleus has distinct motor and non-motor subregions.15,16 The dorsolateral region of the subthalamic nucleus is associated with motor benefit in PD17 and is therefore targeted in the lead implantation. In the case of the globus pallidus internus, the target region is in the posterolateral aspect of the nucleus, with the electrode positioned dorsolaterally relative to the optic tract. Due to its proximity to white matter tracts, minor shifts in the electrode along the x–y axis can cause the VTA to spread outside these nuclei and engender stimulation-induced side effects (Table 1).
Segmented electrodes and steering technology
Current fractionation distributes stimulation disproportionately across a combination of two or more electrode contacts. This approach can be customized using three current fractionation approaches: (1) ‘multi-stim set’ or ‘interleaving’ stimulation, which rapidly alternates between stimulation paradigms that may have different parameters (i.e. amplitude, pulse width) in single-current source systems; (2) multiple independent current control (MICC), which distributes current independently through various electrodes; and (3) coactivation, which offers concurrent activation of multiple electrodes based on a parallel hardware connection, thus enabling each electrode to be treated as an independent contact (Figure 1).18
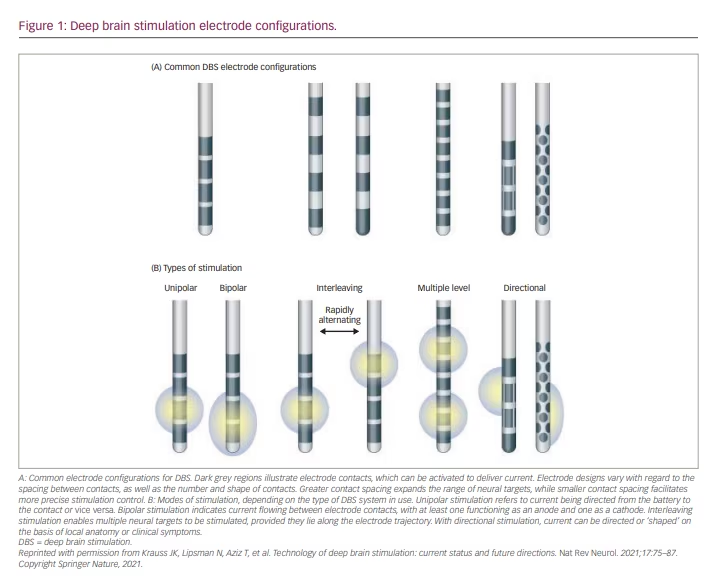
Directional leads consist of two cylindrical contacts and six directional contacts created from segmentation of the middle two contacts of a traditional quadrupolar DBS lead. Each segment is individually spaced 120 degrees apart in the orthogonal plane along the long axis, thus creating a ‘1-3-3-1’ lead design. These leads became commercially available in 2015, with the ability to modulate the VTA such that clinical benefit could be optimized while minimizing stimulation-related side effects.
In the Infinity DBS System (Abbott Laboratories, Chicago, IL, USA) the segments can be activated in parallel, with all receiving the same stimulation parameters. This coactivation approach to current fractionation allows the concert of electrode segments to act in unison – similarly to a single non-segmented electrode. By approaching stimulation in this manner, each stimulating segment increases current with decreased impedance (and therefore theoretically decreased power consumption); however, modulating the VTA is restricted to the segment(s) that are activated.18
The Vercise DBS System (Boston Scientific, Marlborough, MA, USA) employs MICC, which treats each contact as independent from one another. The amplitude of current delivered through any given contact can be modified by the clinician. In this manner, the applied current can stem from multiple contacts, allowing for increased precision in shaping the VTA. MICC technology was demonstrated to be safe and efficacious in the INTREPID trial, which was a double-blind, randomized, sham-controlled trial in patients with PD implanted with subthalamic nucleus DBS.19
The Percept PC system (Medtronic, Inc., Minneapolis, MN, USA) utilizes leads that follow a similar 1-3-3-1 pattern but are also capable of sensing local field potentials – brain oscillations that reflect neural activity from adjacent neuronal elements. Beta band (13–30 Hz) oscillations in the basal ganglia have been shown to be associated with rigidity and bradykinesia in the PD state,20 and suppression of beta activity by DBS is correlated with improvement in these symptoms.21 The ability to record local field potentials from directional leads enables more precise feedback on lead placement, since dDBS leads may rotate or shift following implantation.22 Furthermore, determining the topographical distribution of beta activity associated with motor symptoms may help to identify the appropriate electrode(s) to deliver therapeutic stimulation efficiently while monitoring both clinical response and beta band suppression in real time.
To our knowledge, there has been very little work comparing these stimulation methodologies, with most studies evaluating the efficacy of and differences between these systems relying on computational18,23 or in silico models.24 However, a study from 2020 suggested that coactivation uses less power when compared with MICC.18 These new dDBS systems expand the array of stimulation options, thus increasing the complexity and nuances of the programming that clinicians must now take into consideration.25
Clinical outcomes data for directional deep brain stimulation
In 2014, two intraoperative studies reported on the use of directional leads for the treatment of PD and essential tremor.26,27 These seminal studies found that by modulating the direction of the electrical stimulation, it was possible to achieve diminished stimulation-induced adverse effects by increasing the therapeutic window (i.e. the range from the minimal threshold for clinical benefit and the threshold for the first stimulation-related side effect). However, in terms of elucidating improved clinical outcomes, Contarino et al. did not find any significant change in the suppression of rigidity symptoms.27
Steigerwald et al. investigated the feasibility of current steering with the Vercise system, and showed an expanded therapeutic window as compared with omnidirectional DBS.28 Dembek et al. performed the first prospective, double-blind, randomized controlled trial, in 10 patients.29 The results indicated that stimulation in the optimal direction produced a larger therapeutic window and higher side-effect threshold compared with omnidirectional DBS, along with motor improvements realized 3–6 months after implantation. The VANTAGE study was a first-in-human premarket study.30 This prospective, non-randomized study investigated the implementation of MICC technology in 53 individuals with PD, who were implanted with bilateral subthalamic nucleus DBS with an eight-contact non-segmented electrode. At week 26, patients had a mean reduction of 23.8 in the Unified Parkinson Disease Rating Scale III score, and this was sustained to week 52. Post hoc analysis demonstrated that 72% of the DBS programmes used MICC at week 26 and 68% at week 52. Hence, MICC was found to have an acceptable safety profile and efficacy in PD.
The PROGRESS trial examined the changes in therapeutic window of 234 patients with PD implanted with bilateral segmented electrodes in the subthalamic nucleus.31 Similarly to the other studies, this trial showed expansion of the therapeutic window by an average of 41% in 90.6% of patients when implementing directional stimulation compared with omnidirectional DBS; however, there were no significant improvements in stimulator battery life or motor outcomes.31 Interestingly, the PROGRESS trial did find that both clinicians and patients preferred dDBS over omnidirectional DBS stimulation.31 Ten Brinke et al. similarly reported that providers preferred dDBS over omnidirectional DBS for its improved symptom relief and diminished side effects.25
Challenges and future directions
A major challenge with dDBS is determining segment orientation following implantation. DBS leads can show large deviations from their intended implantation,32 which not only blunts the clinical benefit but, in the case of dDBS leads, can alter the orientation of the segments, thereby invalidating the anatomical location determined by presurgical magnetic resonance imaging (MRI). It is also important to consider the rotational orientation of the dDBS lead, as animal studies have shown that dDBS leads are prone to rotating and shifting following implantation;22 however, a retrospective analysis in humans reported that lead orientation is relatively constant for several weeks.33 Several novel techniques in development, including rotational X-ray,34 magnetoencephalography35 and machine-learning algorithms,36,37 have shown promise in confirming an appropriate lead orientation. The extent of migration of dDBS leads over larger spans of time is still relatively unknown, but the development of techniques that can assist in ensuring knowledge of the proper lead orientation is crucial regardless.
While it can be challenging to confirm proper orientation prior to initiating stimulation, visualization tools allow clinicians to integrate the brain anatomy, usually acquired from the presurgical MRI, and lead location to create VTA models that can inform programming. Medtronic and Boston Scientific have integrated visualization software into their platforms. The Medtronic system incorporates SureTune software, which uses a single brain atlas and homogeneous assumptions in the algorithm to visualize VTA on a co-registered patient-specific MRI.38 The user is also able to adjust the locations, size and shape of the VTA through this platform. Boston Scientific’s Vercise system uses Guide XT visualization software. It creates VTA modelling based on registration of a patient’s postoperative computed tomography images to their segmented presurgical MRI based on an anatomic atlas. The visualization tool enables three-dimensional modelling of the VTA with contouring of the field to be appreciated based on the electrodes or segments activated via MICC.
Visualization approaches using VTA further expand the data-driven evolution of DBS programming, but their limitations must also be contextualized. VTA modelling is based on a presurgical MRI and does not account for post-implantation brain shift or lead deviations.32,39 In addition, VTA models do not take into consideration tissue-specific inhomogeneities, including impedances and local structural lesions.40
When combined with visualization tools and sensing technology, dDBS offers the possibility of probing distinct neural networks, as suggested by a recent study by Peeters et al., which conducted functional analysis of dDBS using electroencephalography.41 This further bolsters the clinical opportunities that will come as adaptive closed-loop DBS emerges.42 Nonetheless, the long-term effects of dDBS compared with conventional leads remain unknown; its therapeutic agency in treating axial symptoms of PD warrants further study; and its applicability with other programming approaches, such as image-guided DBS and artificial intelligence-based programming paradigms, has not been fully investigated.43,44
Conclusions
The emergence of dDBS has heralded a new era in neuromodulation – one that has the potential to expand the benefits for people with PD, optimize clinical outcomes more efficiently and treat refractory symptoms in a data-driven manner. By implementing a multimodal programming strategy of novel current fractionation technology combined with image-guided tools for lead localization and brain sensing, dDBS stands to further diminish the conventional trial-and-error programming approach and usher in a more predictive way to apply this therapy. Such advances will ultimately lead to the development of robust closed-loop stimulation (adaptive DBS) systems that are capable of integrating various streams of continuous data on the disease state.