Treatment of acute central nervous system (CNS) conditions requires effective drugs that can provide rapid onset of effect, consistent blood levels, ease-of-use for patient or caregiver, and acceptable tolerability. Solid oral dosage forms account for up to 75% of prescriptions from healthcare providers.1 They are less expensive and easier to manufacture than drug–device combination products, offer good stability, may be developed with controlled-release properties, are non-invasive, may provide accurate dosing, and are both convenient and easy to use for the patient.1,2 However, oral delivery may result in slow absorption and onset of effect,3 side effects due to high systemic drug levels,4 and possible interactions with food or other orally administered drugs.5 Some CNS disorders, such as migraine, are associated with high rates of nausea and vomiting, that can further impair oral absorption,1,2,6,7 and an often under-appreciated gastrointestinal (GI) dysmotility both within and between migraine episodes.8,9 Further, some oral triptans have lower oral bioavailability ≤50% (eletriptan, frovatriptan, rizatriptan, sumatriptan, zolmitriptan)10 and slow absorption, resulting in delayed peak plasma concentration (Tmax) ranging from 1.2–2.0 hours, and slow onset of action10 or a lack of effect if the “triptan window” is missed.11
Among patients with Parkinson’s disease, gastric stasis often occurs and food intake may interfere with oral medication absorption.12 Indeed, oral dosing may be limited by the concomitant presence of gastroparesis, swallowing difficulties or dysphagia, that occur in up to two-thirds of patients.1,2 Up to one-third of the population experiences swallowing difficulties during their lifetime,2 and even among the general population, 1–16% experience dysphagia, rising to >60% of those in nursing homes who experience difficulty swallowing solid oral dosage forms.6
While injectable formulations may be an option for some CNS disorders, injections are poorly accepted by most patients, especially those with underlying mental illness;13 may weaken patient–physician trust; and may require healthcare professional administration. Further, the need for restraint during administration may lead to nursing staff and patient injuries.14 Thus, a need exists for non-injected, non-oral dosage formulations, with the efficiency of an injectable, as alternatives for treating many acute CNS conditions.
Nasal drug delivery systems for central nervous system disorders
Nasal drug delivery has the potential to provide a more rapid onset of activity; avoids degradation in the GI tract and first-pass hepatic metabolism; is associated with a low risk for GI adverse events; can be administered independent of meals, potentially by a caregiver; and provides patient convenience and ease-of-use.15 Several nasal delivery systems are commercially available with several more, including one targeting the previously unutilized upper nasal space, in clinical development.
In addition, a growing body of research is investigating the possibility of direct access of small-, and especially of large-molecule drugs via olfactory and trigeminal pathways to the CNS using nasal delivery.16,17 This may allow the circumvention of the blood–brain barrier and blood–cerebrospinal fluid barrier.17 The nose-to-brain pathway in animals was first confirmed in 1985 using wheat germ agglutinin-horseradish peroxidase,18 but had been known since the 1930s as the route for poliomyelitis virus ingress,19 later for vesicular stomatitis virus,20 and later still for metals.21 More recently, several early development programs have shown the ability of nasally delivered neural stem cells to migrate through the cribriform plate at the ceiling of the upper nasal space and thence into the olfactory bulb or into the cerebrospinal fluid.22,23 While this research is encouraging, we will limit this review to the emerging clinical data from three different clinical programs delivering established small-molecule CNS drugs to the previously unutilized upper nasal space. Early and consistent systemic blood levels have been reported making this device a potentially useful future option for consideration. Now that systemic blood levels due to delivery to the upper nasal space has been demonstrated, predictably and repeatedly, in humans, the way is open for future non-invasive programs to utilize this route with small- or large-molecule new chemical entities, oligonucleotides, or even cells.
Traditional nasal delivery targeting the lower nasal cavity
The potential advantages of nasal drug delivery to treat acute migraine have been recognized for many years, including avoidance of GI absorption, decreased side effects, and more rapid onset compared with oral medications.24 Several drugs using this route have been approved with more in development, including dihydroergotamine (DHE) mesylate (Migranal® [Bausch Health, Laval, Canada] approved in 1996 and new liquid [INP104] and powder [STS101] products in late clinical development), sumatriptan (Imitrex® [GlaxoSmithKline, Brentford, UK] in 1992, Onzetra® Xsail® [Currax Pharmaceuticals, LLC, Morristown, NJ, USA] in 2016, and Tosymra™ [Upsher-Smith Laboratories, LLC, Maple Grove, MN, USA] in 2019), and zolmitriptan (Zomig® [Amneal Pharmaceuticals, Bridgewater Township, NJ, USA] in 2003). Yet according to the 2009 American Migraine Prevalence and Prevention study, at least 40% of patients have at least one unmet need from their medication, and only ~5.5 million (of the estimated pool of 39 million) patients in the USA receive a regular preventive medicine.25 Migraine remains a major cause of disability and lost productivity, especially for otherwise healthy adults during their most productive years.26
In addition, potential limitations of traditional nasal drug administration include rapid elimination from the nasal space due to dripping from the front of the nose, or down the back of the throat,16 exacerbated by improper administration technique; mucociliary clearance; and lower nasal space architecture. The respiratory mucosa of the lower nasal space is prone to edema and inflammation, or variability in mucous layer cover as a result of infections or allergy, leading to variable drug absorption.16 In addition, the respiratory mucosa may be subject to local discomfort and irritation,15 and drug lost to the nasopharynx can result in taste disturbance.27
Upper nasal drug delivery
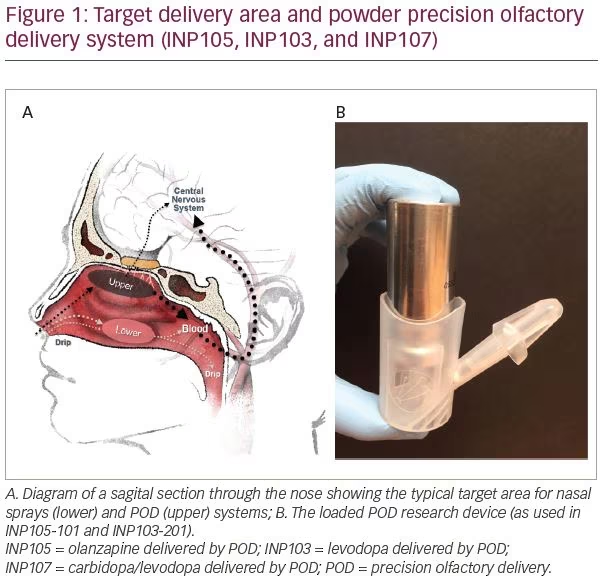
While the nasal space provides an option for delivering drugs to the systemic circulation, most (traditional) nasally administered drugs reach only the vestibule with its squamous epithelium or lower nasal space and turbinates, where absorption via the respiratory epithelium is variable and plasma drug levels are inconsistent.28 Due to its complex architecture, drugs delivered here, by standard nasal devices such as droppers, sprays or pumps, often deposit <5% of active drug into the upper nasal space where absorption is greater, but can sometimes be improved by active, forced sniff.29 Delivery to the olfactory epithelium-lined, highly vascular upper nasal space (Figure 1A) provides faster systemic absorption resulting in therapeutic drug levels and systemic drug effects.28
Precision olfactory delivery
Impel NeuroPharma developed the Precision Olfactory Delivery, or POD®, nasal drug delivery platform, which utilizes the rich vasculature found in the olfactory region of the upper nasal space to provide consistent and predictable drug delivery and improve bioavailability (Figure 1A).28,30 The thickness of the olfactory mucosa in the upper nasal space remains relatively constant to allow the olfactory neurites that penetrate through this mucosa to sense the environment. Drug is less prone to be lost from this space to pharynx or nares; thus consistent drug delivery to this previously overlooked area should provide consistent drug levels and hence clinical response.28,30
The POD system is a handheld, manually-actuated, gas-propelled, administration device designed to deliver active drug specifically to the upper nasal space (Figure 1B). The proprietary nozzle design allows for a narrow-targeted plume to pass beyond the nasal valve to reach the expansive surface area of the upper and middle turbinates and olfactory epithelium. The biphasic emission of the propellant launches the drug, and then pushes it, to the farthest reaches of the nasal space. By maintaining the hydrofluoroalkane propellant and the liquid, or powder drug, separate until the time of delivery, the POD overcomes the manufacturing challenges of maintaining dose uniformity and drug stability when active drug is suspended in the hydrofluoroalkane propellant. The system can overcome the limitations of older nasal delivery systems by utilizing metered, propellant-powered delivery without the need to coordinate breathing or keep the head in a specific orientation, and can be easily administered by the patients themselves, or even by a caregiver. Currently, POD technology is being investigated to deliver DHE,27,31 olanzapine (OLZ),32 and a combination of carbidopa plus levodopa33 to treat acute migraine, acute agitation, and OFF episodes in patients with Parkinson’s disease, respectively.
Clinical data
Acute treatment of migraine
INP104—dihydroergotamine mesylate (in development)
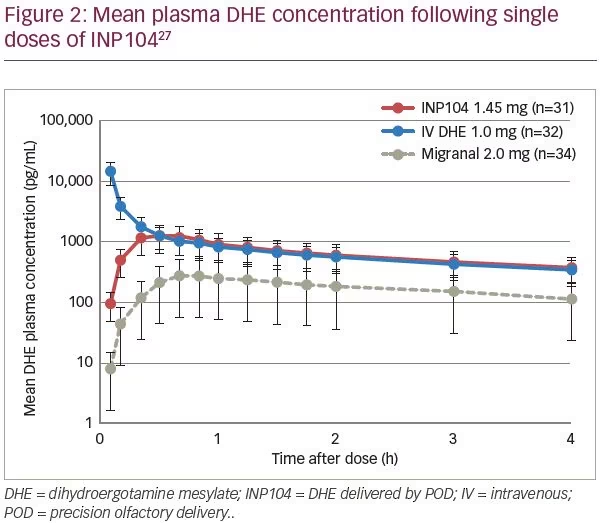
Delivery of DHE using POD technology (INP104) was evaluated in an open-label, randomized, three-period, three-way crossover study, in 38 healthy subjects (ClinicalTrials.gov identifier: NCT03401346).27 Subjects received single doses of INP104 1.45 mg (treatment A), intravenous (IV) DHE 1.0 mg (treatment B), and DHE nasal spray 2.0 mg (treatment C) in one of six sequences (treatments: ABC, ACB, BAC, BCA, CAB, CBA). Whilst INP104 avoided the undesirable high peak plasma DHE concentration seen with IV administration, it generated similar plasma levels by 20 minutes and comparable exposure (area under the curve [AUC]) (Figure 2).27 INP104 achieved a four-fold increase in DHE Cmax, and a three-fold increase in DHE AUC compared with liquid DHE nasal spray (Migranal), using the identical formulation but <75% of the dose. Variability in Cmax and AUC was substantially reduced with INP104. The incidence of any treatment-emergent adverse event was 19.4% with INP104, 34.4% with IV DHE, and 11.8% with DHE nasal spray. Treatment-related nausea was only reported with IV DHE (three subjects) and DHE nasal spray (one subject), despite metoclopramide premedication. Mild nasal discomfort occurred in one subject each with INP104 and liquid DHE nasal spray. Drug leakage from the nose or into the nasopharynx was reported by 32.3% with INP104, and 76.5% with nasal spray. INP104 provides a novel nasal delivery system with a pharmacokinetic (PK) profile that is comparable to IV DHE from 20 minutes, with greater consistency than the nasal spray and in an easy-to-use system.
Results from a 360-patient phase III study with INP104 (STOP-301; ClinicalTrials.gov identifier: NCT03557333), assessing the safety and tolerability of chronic, intermittent use over 24 weeks, with a subset over 52 weeks, are expected in 2020.31 Nasal mucosal integrity and olfactory function are being evaluated with upper nasal endoscopy and the University of Pennsylvania Smell Identification Test (UPSIT), respectively. STOP-301, enrolling patients with a mean baseline migraine frequency of 4.7 episodes/month, is an open-label safety study utilizing a daily e-diary to assess exploratory efficacy, healthcare utilization and quality of life, and will represent the largest dataset of repeat, long-term DHE use in acute migraine when published. The data was collected both during the 28-day screening period, as well as for every day and with every migraine during the 24- or 52-week treatment periods.
Interim data on the acceptability of the product,34 reported results for a series of questions asked of 164 subjects who had then completed the 24-week study. Patients reported agreement or strong agreement that INP104 was easy to use in 90% of cases. Other questions asked were: Did INP104 allow you to return to normal faster? Did INP104 consistently treat your migraines? Did INP104 work faster than your previous treatment? Did INP104 stop your migraines from coming back? Was INP104 easy to use? Answers to these questions were all scored: Strongly agree; Agree; Neutral; Disagree; or Strongly disagree, and all provided encouraging data.
Acute agitation
INP105—olanzapine (in development)
Acute agitation is common in patients with schizophrenia and bipolar disorder,35 as well as other mental health disorders such as post-traumatic stress disorder, Alzheimer’s dementia, and neurodevelopmental conditions, for example, Down’s syndrome or autism-spectrum disorder, where it may be referred to as “irritability”.36 OLZ intramuscular (IM) is often used to treat acute agitation because of a shorter Tmax, leading to more rapid onset of efficacy than oral administration, and a lower risk of extrapyramidal adverse effects than first-generation antipsychotics, but requires patient cooperation or restraint, is invasive, and can be painful.14 Injection may risk injury, loss of doctor–patient trust, and result in psychiatric boarding; whereas oral products have slower onset of effect, often requiring observation of the medicated patient for a sustained period of time, until their agitation resolves,36 which may be impractical. While non-pharmacological management is preferred, access to the appropriate staff to conduct it, time and location to deliver it, and other operational issues often make it hard to deliver in practice, and effective, rapid-acting, non-injected options are lacking.
In 2018, the Centers for Disease Control and Prevention determined that autism spectrum disorder may affect 1 in 37 boys and 1 in 151 girls.37 There are currently two oral antipsychotics approved for autism-associated agitation and irritability, yet interestingly children with autism have an eight-fold higher chance of chronic GI disorders,38 and chronic dosing with these drugs to reduce the incidence of agitation is hampered by tolerability issues. A rapidly-effective therapy for relief of agitation or irritability in these patients is much needed, especially non-oral.
Precision olfactory delivery olanzapine (in development)
A nasally-administered, fast-acting, well-tolerated, OLZ formulation would provide an alternative to IM or oral forms. INP105 is a drug–device combination product consisting of a powder formulation of OLZ delivered by POD32 at a dose of 5 mg with each actuation, taking approximately one-tenth of a second. INP105 offers non-invasive delivery of OLZ into the upper nasal space enabling rapid systemic absorption without an injection.
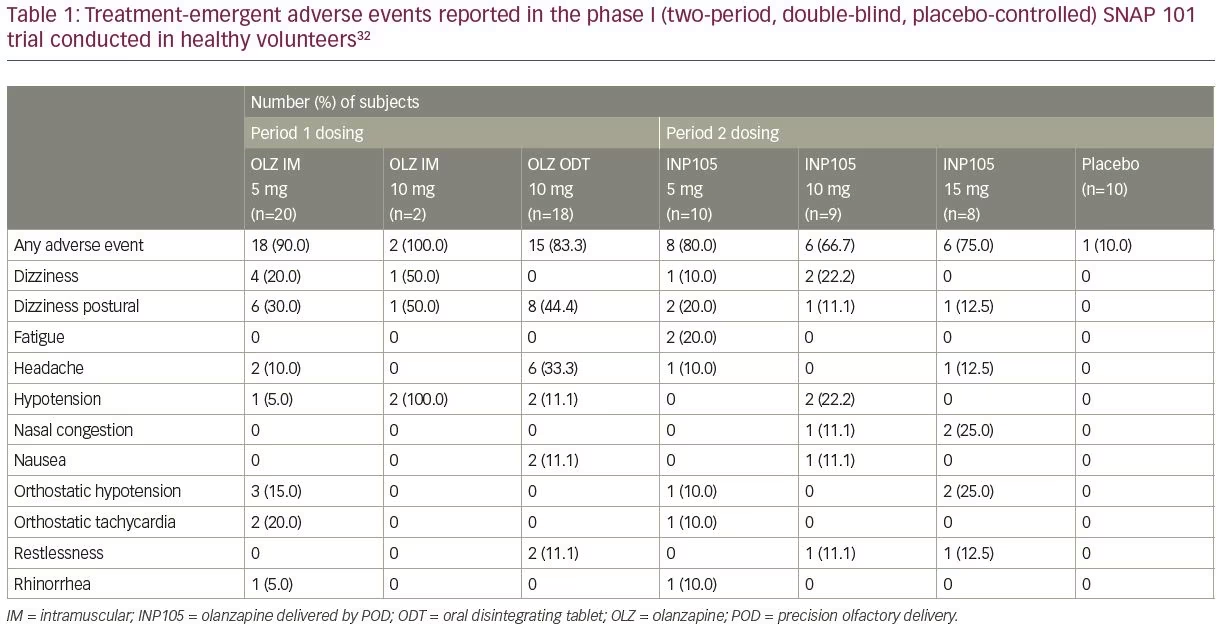
SNAP 101 (INP105-101; ClinicalTrials.gov identifier: NCT03624322) was a randomized, placebo-controlled, two-period crossover study to evaluate the safety and tolerability of INP105.32 Three single ascending doses
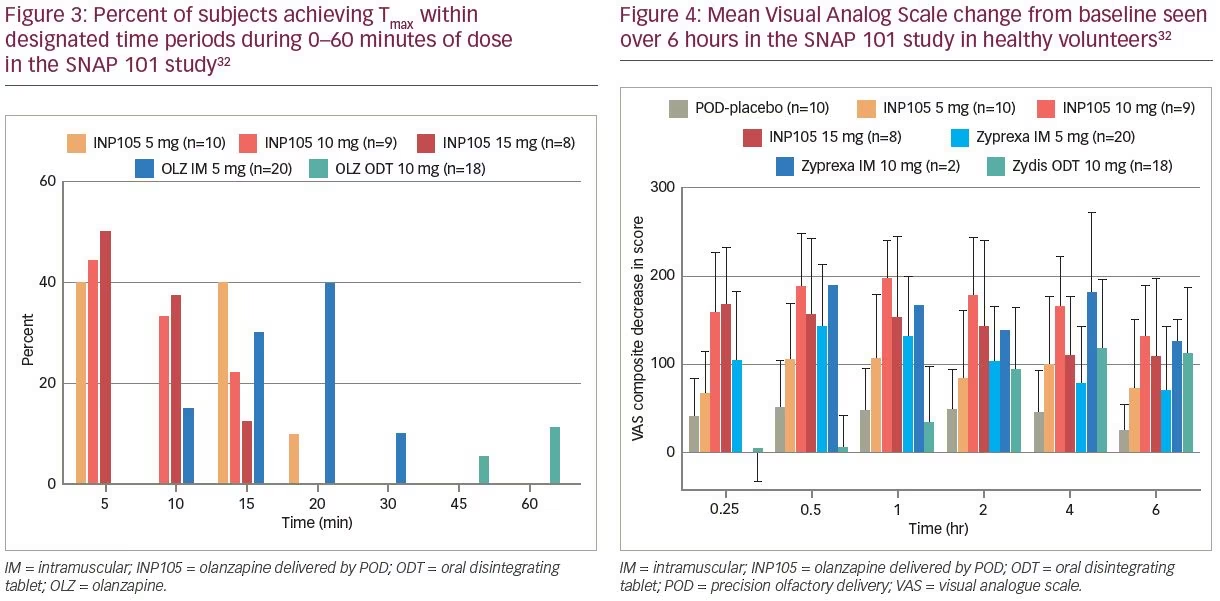
(5, 10 and 15 mg) of INP105 were administered by the POD research device (Figure 1B) to compare the PK and pharmacodynamic effects of INP105 with OLZ IM (5 and 10 mg), OLZ oral disintegrating tablet (ODT) 10 mg, and placebo. It was not conducted in patients suffering from agitation, as the plan for development of INP105 is to “bridge” the PK data in healthy volunteers to that of IM OLZ, and if successful, obviating the need to conduct an efficacy trial. INP105 was well tolerated after a single dose (Table 1) in healthy volunteers who had received either OLZ IM or OLZ ODT at least 14 days earlier in period 1. OLZ exposure with INP105 5 mg closely matched OLZ IM 5 mg, with most individual peak plasma levels occurring within 5–15 minutes with INP105 versus 10–20 minutes with OLZ IM 5 mg (Figure 3).32 Clinically meaningful calming effects were observed with INP105 versus placebo based on:
1) The Agitation and Calmness Evaluation Scale with changes of up to ~2 points over the first hour with INP105 that matched or exceeded the changes seen with OLZ IM 5 mg, whereas OLZ ODT 10 mg and placebo did not substantially change from baseline in this first hour. The maximum changes over 6 hours were 0.6 (placebo), 2.1 (INP105 5 mg), 2.9 (INP105 10 mg), 2.8 (INP105 15 mg), 1.9 (OLZ IM 5 mg), 2.8 (OLZ IM 10 mg) and 2.0 (OLZ ODT 10 mg).
2) The combined 300 mm Visual Analog Scale (exploring alert/drowsy; foggy/clear-headed and energetic/lethargic) showed rapid onset of effect with all three INP105 doses (of similar magnitude to OLZ 5 mg IM), substantially earlier than OLZ ODT and greater at all timepoints (out to 6 hours) compared with placebo (Figure 4).
3) The Digit Symbol Substitution Test (DSST) assessed response speed, sustained attention, visual spatial skills, and set shifting that corresponded to a series of digits (numerals) outlined on a test paper. Subjects were given 90 seconds to record the symbols associated with each numeral and the number of correctly substituted digits noted. There was a dose-dependent, desired, decrease in mean DSST scores representing a slowed response speed over the first 6 hours of testing for all doses of INP105 compared with placebo (p<0.01). For INP105 5 mg and 10 mg score changes were comparable to OLZ IM 5 mg and greater than OLZ ODT 10 mg. Changes were maximal at
30 minutes (for 10 and 15 mg) and sustained for up to 6 hours. DSST score decreased at 2, 4, and 6 hours compared to placebo with OLZ ODT (but were not observed in the first hour with the ODT).
Thus, INP105 may represent a needle-free alternative for treating acutely agitated patients and could become a valuable option for self or caregiver administration in the psychiatric ward, nursing homes, the community (self-administered by self-aware patients) or the home environment.
Parkinson’s disease—OFF episodes
Patients with Parkinson’s disease experience fluctuations of motor symptoms, even with optimized treatment. While levodopa is a standard of care for managing the dopamine-related symptoms of Parkinson’s disease, most patients develop motor complications related to fluctuations in the levels of plasma levodopa over the course of their disease that may become debilitating and impact quality of life. An unmet need exists for rescue therapy that will rapidly and efficiently reverse OFF periods, especially in the early morning when the benefit of the previous evening’s medication is at a nadir.
POD levodopa and POD carbidopa/levodopa (in development)
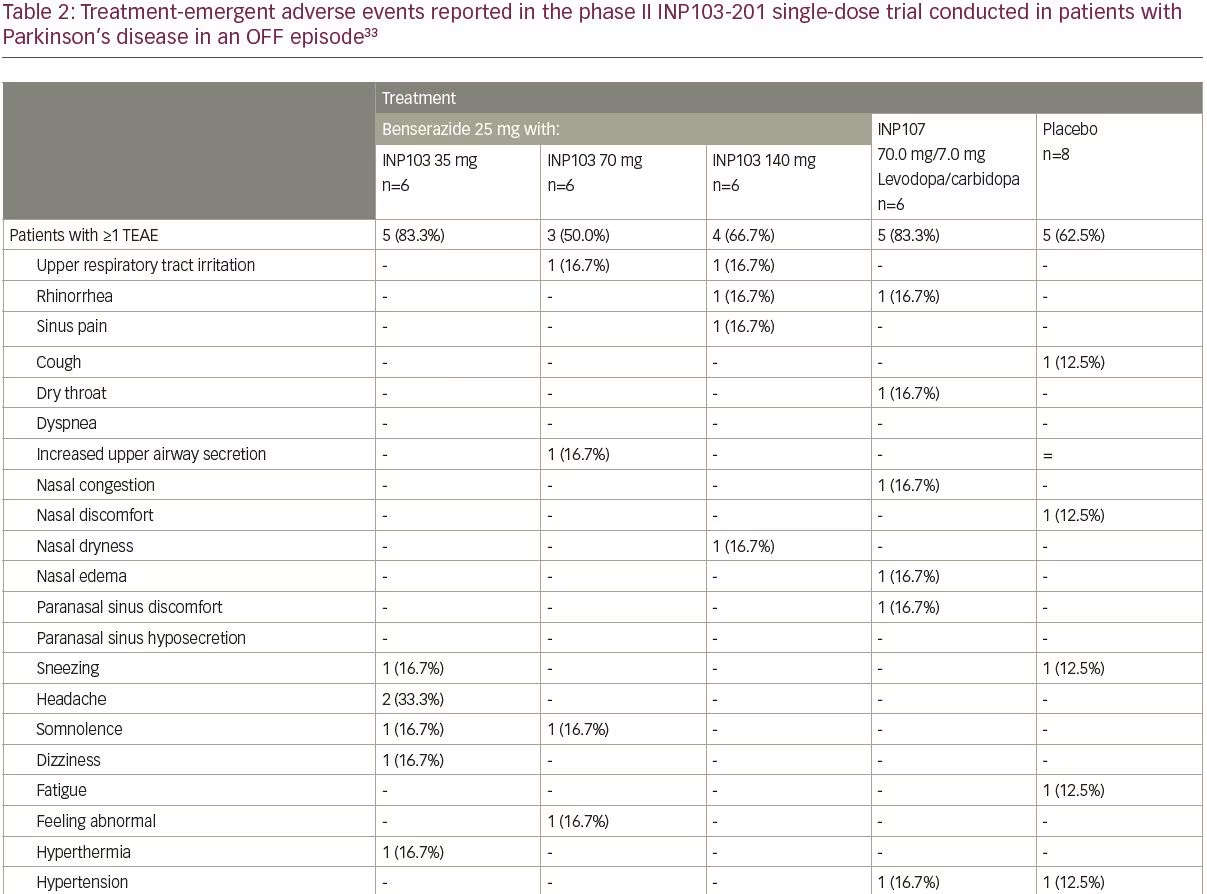
INP103 is a drug–device combination product containing levodopa delivered by the POD device. THOR 201 (INP103-201; ClinicalTrials.gov identifier: NCT03541356) was a randomized, double-blind, placebo-controlled, single-dose study that assessed the safety, tolerability, PK and pharmacodynamics of levodopa 35, 70, and 140 mg administered by the POD device with a dopa decarboxylase inhibitor (DCI), for the treatment of early morning OFF episodes of Parkinson’s disease.33 This was a proof of concept trial, albeit conducted in patients with Parkinson’s disease, to ascertain if sufficient systemic levels of levodopa could be achieved to reverse OFF episodes. In the first three cohorts of eight subjects each (six active, two placebo), oral benserazide 25 mg DCI was dosed 60 minutes before INP103. As pharmaceutical development work progressed in parallel, a fourth cohort was added in early 2019, with INP107 in a 10:1 combination of levodopa/carbidopa at a dose of 70.0/7.0 mg. The levodopa absorption following early morning nasal administration of INP103 35, 70, and 140 mg resulted in a median Tmax ranging between 45.5 and 60.5 minutes depending on dose, and 90.5 minutes for the combined carbidopa/levodopa (INP107) formulation. These corresponded to mean Cmax levels of levodopa of 294, 394 and 676 ng/mL for INP103 and 456 ng/mL for INP107.
Adverse events occurred at comparable rates among all dose cohorts, and all adverse events were mild (Table 2), except for one moderate treatment-emergent adverse event of urinary tract infection with no specific event reported by more than one subject in any cohort. Abnormal nasal examination findings were infrequently observed and were mild in severity. Most patients found the POD device comfortable to use and preferable compared with their current drug delivery method. The absorption of levodopa from the upper nasal space yielded adequate systemic exposure, which at 400 ng/mL, has reversed daytime OFF motor symptoms,39 but the time to reach those concentrations was longer than desired and further work varying the ratio of carbidopa to levodopa is expected to greatly reduce that, optimize the PK profile, and provide clinical benefit, even for early morning OFF episodes, where absolute levodopa needs may be in the range 800–1,100 ng/mL.40 The efficacy assessments, using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), revealed mixed results with INP103, INP107 and placebo, and given the PK results (suggesting, at lower dose, the systemic levodopa level would not be expected to reverse morning OFF), small sample size, and 3:1 active:placebo randomization, may not represent a true pharmacological response.
Summary
While most drugs are administered as oral tablets, capsules or solutions, the need for rapid, effective systemic levels in disabling acute disorders, such as migraine, emergency situations (acute agitation), or in conditions affecting cognition and function (Parkinson’s disease), make an easy to administer, non-injected, non-oral therapy much desired. In addition, the effectiveness of oral forms may be limited in patients suffering from dysphagia or GI motility problems, which are prevalent in migraine and Parkinson’s disease. While a few drug–device products are approved for use in the USA to treat CNS disorders, the novel POD system is in clinical development for acute migraine, acute agitation, and OFF episodes in Parkinson’s disease. The non-invasive POD system provides the most efficient nasal delivery of drugs allowing it to outperform traditional nasal delivery products, as well as IM injection, with an optimized dose of active drug that results in few side effects. Delivery of drugs to the upper nasal space provides a useful, well-tolerated, and reliable alternative and may be a welcome addition to the management of many CNS disorders.