Pathologically, Parkinson’s disease (PD) is characterized by the loss of dopaminergic neurons in the substantia nigra and the presence of Lewy bodies.1 In a staging model proposed by Braak in 2003,2 the presence of midbrain lesions are preceded by degeneration and Lewy bodies in lower brain stem nuclei. Indeed, non-motor features such as constipation, sleep disorders, hyposmia, and depression may precede motor symptoms by years and even decades (Figure 1).3 Throughout the course of PD, it has become increasingly recognized that non-motor symptoms continue to occur as motor symptoms progress.3

Of the one million people in the US who live with PD,4 more than 50% will develop psychosis during the course of their disease.5 Parkinson’s disease psychosis (PDP) is clinically distinct from other psychotic disorders such as schizophrenia, and is characterized by hallucinations6–8 and delusions.6–9 Visual hallucinations – including presence or passage hallucinations – or illusions are most common. It is common for patients to report seeing animals or children (see case study). Tactile hallucinations may accompany visual hallucinations; other types include auditory (e.g., music, indistinct voices), olfactory, somatic and gustatory hallucinations.6–8 Delusions frequently include beliefs of marital infidelity and relatives stealing money.7,9–11
__________________________________________________________________
| Presentation and medical history
|
__________________________________________________________________
This article reports on an Industry Therapeutic Update (sponsored by ACADIA Pharmaceuticals Inc.) which was held at the 69th Annual American Academy of Neurology (AAN) Meeting (April 22–28, 2017) in Boston, MA, USA. The objectives of this Industry Therapeutic Update were to explore the pathophysiology, symptomology, diagnosis and impact of PDP, the role of serotonin signaling within PDP, and to examine the current standard of care for treatment for the condition. In addition, the proposed mechanism of action, efficacy, safety, and administration of pimavanserin as a treatment for hallucinations and delusions associated with PDP will be summarized.
Diagnostic issues
“PDP is far more common than many of our patients are aware; they need to know that this is part of the underlying disease.” – Stuart Isaacson
PDP can be diagnosed according to the 2007 National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Mental Health (NIMH) provisional diagnostic criteria.6 Diagnosis of PDP requires the presence of at least one of the following symptoms: hallucinations, illusions (i.e., misperception of actual stimuli in contrast to hallucinations which occur in the absence of real stimuli), delusions, or false sense of presence.6 Associated features that may or may not occur in PDP include insight, dementia, and the patient may or may not be receiving drugs for treatment of PD. Symptoms must occur in patients with previously diagnosed PD and must be recurrent or continuous for at least 1 month. Other causes should be excluded, including delirium, dementia with Lewy bodies, and psychiatric disorders such as schizophrenia, schizoaffective disorder, delusional disorder or mood disorders with psychotic features.6
A validated, patient self-report questionnaire (NMSQuest) was used in a study (n=242) designed to identify the non-motor symptoms – including delusions and hallucinations – that may not have been disclosed to physicians.12 Of the patients who completed the surveys and experienced symptoms, 41.5% experienced hallucinations and 65.2% experienced delusions they did not declare to their physicians. Patients may under-report PDP symptoms for a variety of reasons, including the fact that it may be unclear to patients that the non-motor symptoms they are experiencing are related to PD, and during time-restricted consultations, patients may simply not have enough time or forget to mention non-motor symptoms.12,13
__________________________________________________________________
| Symptoms of Parkinson’s disease psychosis
|
__________________________________________________________________
PDP often begins with hallucinations with retained insight13 and, while the patients remain cognizant of these PDP symptoms, they are often able to cope without behavioral disturbances.14 However, as PDP progresses, insight is often lost and delusions may occur;6,13,15 patients may find these symptoms threatening and may respond or act aggressively, creating behavioral disturbances.16 The majority of patients experiencing mild hallucinations progress to more severe symptoms;13 additionally, caregiver burden increases.17,18 In a case study review of 143 patients with PD who were admitted to a hospital, symptoms of psychosis accounted for 24% of admissions.19 Further, a 4-year prospective study carried out in Norway (n=178) identified that patients with PD experiencing hallucinations were two and a half times more likely to be admitted into a nursing home than patients with PD without hallucinations.20 In addition, mortality at 3 years was approximately 40% for patients with PD and symptoms of psychosis, based on an age-adjusted model with a baseline age of 75 years (n=230).21
__________________________________________________________________
| Progression of Parkinson’s disease psychosis
|
__________________________________________________________________
Both intrinsic and extrinsic factors contribute to PDP. Intrinsic factors include those associated with disease progression (older age, PD severity and duration),22 abnormalities of neurodegeneration (neurochemical abnormalities and visual processing deficits),22–24 comorbid medical conditions (e.g., urinary tract infections [UTIs]) and/or psychiatric conditions.6,22 Extrinsic factors encompass anti-Parkinson drugs (e.g., higher levodopa dose, dopamine agonists) and other medications (e.g., anticholinergics),6,22 and environmental factors (time of day and dim lighting).6,25,26
Pathophysiology of Parkinson’s disease psychosis
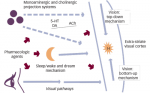
Emerging evidence – albeit from very small studies – indicates that psychosis can result from overactivation of both dopaminergic and serotonergic signaling.1,23,27–29 In an imaging study of five patients with PD and eight control subjects, the patients with PD showed reduced serotonin transporter binding, with the greatest reductions in the forebrain.28 A pilot study was conducted using the selective serotonin 2A receptor (5-HT2A) ligand [18F]-setoperone during positron emission tomography of seven patients with PD and visual hallucinations, and seven age-matched patients with PD but not visual hallucinations.23 Patients with PD and hallucinations were found to have increased 5-HT2A receptor binding in several regions of the brain, including the ventral visual pathway.23 In addition, results from the Parkinson’s Progression Markers Initiative showed that patients (n=21) with early onset formed hallucinations had reduced baseline higher visual function and cortical thinning in the parietal, occipital and frontal cortex, as well as reduced hippocampal volume, compared with the overall PD cohort.30 A challenge for clinicians treating patients for PD is that dopaminergic therapies for motor symptoms typically worsen psychosis,22 but the reduction of anti-Parkinson medication to treat PDP can worsen motor symptoms.31,32
“These studies provide an evidence base that, by modulating serotonin, we might be able to improve the psychotic symptoms of hallucinations and delusions in PD. It is important to consider this new way of thinking about the genesis of PDP because it opens the door to a way of treating PDP without worsening motor symptoms.”– Stuart Isaacson
Treating Parkinson’s disease psychosis
“It’s a real paradigm shift in that we finally have an FDA-approved medication to treat PDP that does not worsen motor symptoms…” – Daniel Kremens
Pimavanserin (NUPLAZID®; ACADIA Pharmaceuticals Inc., San Diego, CA, USA) – which is an atypical antipsychotic – does not worsen motor symptoms, and is the first and only Food and Drug Administration (FDA)-approved treatment for hallucinations and delusions associated with PDP.33 As with all drugs in this class, pimavanserin has a boxed warning regarding mortality in elderly patients with dementia-related psychosis.33
In vivo, pimavanserin binds to 5-HT2A receptors with a five-fold higher selectivity over 5-HT2C receptors. It has no appreciable affinity to dopaminergic receptors (including D2) or to muscarinic, histaminergic, or adrenergic receptors.33 Pimavanserin’s mechanism of action in the treatment of hallucinations and delusions associated with PDP is unknown although it may be mediated through a combination of inverse agonist and antagonist activity at serotonin 5-HT2A receptors, and to a lesser extent, at serotonin 5-HT2C receptors.33
__________________________________________________________________
| Treatment of Parkinson’s disease psychosis
|
__________________________________________________________________
Pimavanserin pivotal phase 3 clinical study
A placebo-controlled, randomized, parallel-group study of 199 patients with PDP was conducted to determine whether pimavanserin can reduce hallucinations and delusions associated with PDP effectively.34 Eligible participants entered a 2-week nonpharmacological lead-in phase, in which patients received brief psychological therapy adapted for PD. Subsequently, patients were randomly allocated (1:1) to receive pimavanserin 34 mg per day or matched placebo for 6 weeks. The primary outcome was antipsychotic benefit as assessed by central, independent raters with the Parkinson’s disease-adapted scale for assessment of positive symptoms (SAPS-PD). The SAPS-PD is a nine-item scale, rated from 0 to 5, that includes hallucinations (five items) and delusions (four items) (Figure 2).9 A 2.33-point change on the SAPS-PD is associated with a 1.00-point change on the Clinical Global Impression-Improvement scale (CGI-I)9 indicating that a 3.00-point improvement on the SAPS-PD can mean the difference between daily and occasional symptoms of psychosis.35 The secondary endpoint was change in the Unified Parkinson’s Disease Rating Scale (UPDRS) Parts II + III.33,34
Key inclusion criteria were: age ≥40 years; PD diagnosis ≥1 year; psychotic symptoms occurred following PD onset, ≥1 month’s duration, occurred at least weekly in the month before screening, and were frequent and severe enough to warrant treatment; stable on PD medication for at least 30 days prior to study start and throughout the 6-week study; neuropsychiatric inventory ≥6 or a score of ≥4 on either the Hallucinations or Delusions domain (at screening); and ≥3 on the SAPS-hallucination or -delusions global item and ≥3 on at least one other non-global item on the SAPS-PD (at baseline).33,34 Patients with delirium or a mini-mental status exam (MMSE36) <21 points were excluded.33,34 Other exclusion criteria were psychosis secondary to toxic or metabolic disorder, psychosis following ablative stereotaxic surgery, presence of dementia concurrent or before PD, and uncontrolled serious medical illness.33,34 In addition, antipsychotic drugs, centrally acting anticholinergics, or drugs that cause QT prolongation were prohibited.33,34

Results
Pimavanserin effectively diminished symptoms of hallucinations and delusions associated with PDP at 6 weeks (Figure 3).33,34 The mean SAPS-PD baseline score for pimavanserin 34 mg was 15.9 and 14.7 for placebo.33,34 For the primary endpoint, the point reduction on the SAPS-PD was -5.79 pimavanserin 34 mg and -2.73 placebo (p=0.0014).33,34 Patients who received pimavanserin had a mean change that resulted in a 37% improvement from baseline compared with 14% for placebo.34 The positive effect of pimavanserin on SAPS-PD improved as early as 2 weeks from baseline after starting treatment, with statistical significance seen at 4 and 6 weeks.34 In an exploratory analysis, pimavanserin also reduced the individual hallucination and delusion domain scores of the SAPS-PD compared with placebo.33 In total, 14 patients discontinued treatment after randomization but prior to the first visit post-baseline, resulting in a primary efficacy analysis population of 185. The mean age was 72.4 years for both pimavanserin 34 mg (n=95) and placebo (n=90).34
Approximately 65% of patients receiving pimavanserin experienced at least a 3-point reduction in hallucinations and delusions associated with PDP as measured by the total SAPS-PD score.33 Illustrated in the case study of Maria (described previously), these improvements can be understood in terms of Maria being unsure whether or not she sees a black cat, and seeing a cat every day and buying food for it. Further, nearly 14% of patients taking pimavanserin experienced a complete response in hallucinations and delusions associated with PDP versus just 1% of patients taking placebo.33 The change from baseline in UPDRS Parts II + III was similar between the pimavanserin 34 mg (n=92) and placebo groups (n=88; -1.4 and -1.7 respectively), indicating that there was no motor worsening associated with treatment.33


Overall, pimavanserin was well tolerated in the 6-week placebo-controlled trials, with the most common adverse events being peripheral edema and confused state (see full adverse events in Table 1). A total of 8% (16/202) of pimavanserin 34 mg-treated patients and 4% (10/231) of placebo-treated patients discontinued because of adverse reactions. The adverse reactions that occurred in more than one patient and with an incidence at least twice that of placebo were: hallucinations (2% pimavanserin 34 mg versus <1% placebo), UTIs (1% pimavanserin 34 mg versus <1% placebo), and fatigue (1% pimavanserin 34 mg versus 0% placebo).33
Clinical program for pimavanserin
“This drug has what has been termed cumulative pharmacokinetics in that a steady state in Cmax is gradually achieved around 10–12 days. When you begin pimavanserin 34 mg/day without titration, as per label, you can reach the steady state that has been associated in the clinical trial with clinical efficacy and tolerability… In a sense, it self-titrates by gradually increasing its concentration in the plasma before reaching steady state.” – Stuart Isaacson

Across the clinical trial program, more than 1,200 people have been exposed to at least one oral dose of pimavanserin; 616 were patients with hallucinations and delusions associated with PDP.33 Additional clinical trial experience in patients with hallucinations and delusions associated with PDP is derived from two open-label, safety extension studies (n=497).33 As of April 2016, more than 300 patients have been treated for >6 months; >270 have been treated for at least 12 months; and >150 have been treated for at least 24 months.33
The clinical trial program included a pharmacokinetic study (Figure 4), which found that the half-life was 57 hours, whereas the time to reach maximum plasma concentration was 6 hours (range 4–24).33 A high-fat meal had no significant effect on rate and extent of pimavanserin exposure.33 Concomitant use with drugs known to prolong the QT interval, including Class 1A or Class 3 antiarrhythmics (i.e., quinidine, procainamide, disopyramide, amiodarone, sotalol), certain antipsychotics (i.e., ziprasidone, chlorpromazine, thioridazine), and certain antibiotics (i.e., gatifloxacin, moxifloxacin), should be avoided as they may add to the QT effects of pimavanserin and increase the risk of cardiac arrhythmia.33 CYP3A4 is the major enzyme responsible for pimavanserin metabolism and pimavanserin can interact with strong CYP3A4 inhibitors and CYP3A4 inducers. Examples of strong CYP3A4 inhibitors are: itraconazole, ketoconazole, clarithromycin, and indinavir, and strong CYP3A4 inducers include: rifampin, carbamazepine, phenytoin, and St. John’s wort.33 The recommended dose of pimavanserin is 34 mg once daily (any time of day), without titration; pimavanserin can be taken with or without food.33 However, the recommended dose of pimavanserin when co-administered with a strong CYP3A4 inhibitor is 17 mg, taken orally as one tablet once daily.33 When pimavanserin is co-administered with a strong CYP3A4 inducer, patients should be monitored for reduced efficacy and the dosage increased if required.33 Based on pharmacokinetic studies, no dosage adjustment of carbidopa/levodopa is required when administered concomitantly with pimavanserin.33
Use in elderly populations
In the 6-week, placebo-controlled studies for pimavanserin, patients up to 90 years were included;35 49% of the patients were 65–75 years old and 31% were >75 years old.33 Patients were required to be 40 years of age or more for inclusion, as well as have an MMSE score greater than 21 so that patients were able to self-report symptoms.33 In the pooled population of patients enrolled in 6-week, placebo-controlled studies (n=614), 27% had MMSE scores of 21–24 compared with 73% with scores ≥25.33 No clinically meaningful differences in safety or effectiveness were noted between the two groups.33 Thus, no dose adjustment is necessary for elderly patients.
Conclusions
More than 50% of patients with PD develop PDP at some point during the course of PD, and the symptoms generally worsen with time.5,13 Evidence suggests the symptoms of psychosis can be the result of excessive serotonergic activity in the raphe nuclei.1,27,29 Pimavanserin is the only FDA-approved treatment for hallucinations and delusions associated with PDP.33 Treatment with pimavanserin reduces hallucinations and delusions in patients with PDP without impacting motor function.33 The recommended dose of pimavanserin is 34 mg, once daily, without titration and without a dosage adjustment in concomitant carbidopa/levodopa.30