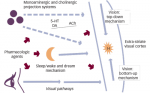
Humans afflicted with narcolepsy suffer a variety of symptoms due to the dysregulation of the REM cycles.3-5 These irregularities cause EDS, leading to uncontrollable urges to sleep known as sleep attacks even in active daily events. This sudden onset of sleep occurs primarily when participation level is low (watching television, reading, sitting in class, etc.).1 While the regular REM cycle causes temporary inhibition of muscles during sleep, narcoleptics experience this temporary feeling of paralysis outside of sleep in processes known as sleep paralysis or cataplexy.1,2,5 Hypnagogic hallucinations often occur in the transition between waking and sleeping, usually in the form of visual images but often in the shape of voices or bodily sensations.2 These symptoms radically affect the normalcy of life for narcoleptic patients; unfortunately, treatment options are still limited. However, chosen wisely, treatment can dramatically increase the quality of life in patients with narcolepsy.
Treatments
Although the disorder was traditionally treated with amphetamines, the last decade has seen US Food and Drug Administration (FDA) approval of modafinil, the first drug therapy specifically approved to treat EDS caused by narcolepsy.5-7 Modafinil is pharmacologically and clinically unrelated to amphetamines.7,8 French pharmaceutical company Laboratoire L Lafon originally developed modafinil and it quickly became the most often prescribed medication to treat EDS in narcolepsy patients.4,5 Modafinil is a non-traditional stimulant whose mechanism of action is not yet entirely understood, although much has been discovered in recent years. Modafinil does not require the norepinephrine transporter or postsynaptic dopamine receptors. Modafinil does bind to the dopamine transporter (DAT), but with low affinity, which has led to the discussion of its relevance. 7 The high plasma concentration of modafinil appears to make up for its low affinity to DAT. However, modafinil is active in areas with both DAT and alpha-adrenergic receptors such as the basal forebrain, cerebral cortex, and the thalamus.9,10 This may explain why modafinil is not useful for the cataplexy symptoms that often accompany narcolepsy, as regions that control muscle tone are not rich in DAT-bearing dopaminergic terminals. In summary, modafinil binds to DAT, which increases extracellular dopamine. Dopamine binds to alpha-adrenergic receptors, which activates downstream; this may cause an increase in dopamine and indirectly increase both histamine and glutamate, all of which may account for modafinil’s wake-promoting properties.11 New findings also point to possible involvement of the endocannabinoid system. Researchers from Mexico City have preliminary data that suggest that modafinil may modulate waking via the CB1 or endocannabinoid receptors.9 Clearly, there is additional work to be undertaken to fully elucidate modafinil s mechanism of action.
In addition to treating the EDS associated with narcolepsy, the FDA has approved modafinil for shift-work sleep disorder and as an adjunctive treatment for obstructive sleep apnea. There is much literature published on other uses for this interesting compound, most of which revolves around treating fatigue and/or sleepiness associated with chronic medical and psychiatric conditions.12
The incidence of significant side effects with modafinil is low, although a small percentage reported headache and nausea,4 which appears to be dose-dependent. Broughton et al. conclude that a dosage of as little as 200mg daily of modafinil was effective in the treatment of EDS for narcoleptic patients.6 Patients who are prescribed modafinil for narcolepsy are instructed to take between 200 and 400mg daily. Its half-life is 15 hours,13 so once-daily dosing is sufficient for most patients.
In comparison with amphetamines, modafinil has not been shown to produce unwanted side effects such as hypertension, disruptive night-time sleep, restlessness, or arrhythmias,4 although it should be noted that in a retrospective analysis of the use of antihypertensive medications, a greater proportion of patients taking modafinil required new or increased use of antihypertensive medications (2.4%) versus patients taking placebo (0.7%).13 Therefore, monitoring of blood pressure may be appropriate for patients taking modafinil. It has very little risk of dependency or abuse,6,7 although there are a handful of reports of sporadic modafinil abuse.14 One case study of a schizophrenic patient suggests the possible exacerbation of psychosis through the prescription of modafinil, although this possibility has not been confirmed.15Modafinil may be combined with antidepressants (clomipramine, protryptiline) for patients suffering from narcolepsy with cataplexy.
Despite modafinil’s popularity, many still treat the symptoms of narcolepsy with the use of amphetamines,4,5 in spite of the increased risk of abuse and shorter duration of action. Aside from potential recreational abuse, patients can develop a tolerance to the stimulants and their dosage may need to be increased to effectively treat EDS.2-5 These medications are highly controlled by agencies such as the Drug Enforcement Administration (DEA). The mechanism of action of amphetamines revolves around manipulation of dopamine. Amphetamine binds to the dopamine transporter and prevents it from taking up dopamine. However, unlike methyphenidate, amphetamine itself is taken up into the neuron via the DAT. Amphetamine also binds to the vesicular monoamine transporter (VMAT). Competitive inhibition of dopamine at the VMAT and transport of amphetamine into the synaptic vesicles causes displacement of dopamine from synaptic vesicles. Displacement of dopamine from synaptic vesicles causes intracellular dopamine to accumulate, leading channels to open and release dopamine into the synapse. It also causes a reversal of DAT, so that dopamine is pumped out of the neuron instead of into the neuron. Therefore, amphetamines are not only DA re-uptake blockers, but DA releasers. 11,16
Amphetamines are usually prescribed in amounts ranging from only 5 to 20mg/twice a day, but can be increased due to the severity of the symptoms.4
Due to their intense stimulation of dopaminergic tone, amphetamines can cause severe side effects in the form of agitation, confusion, anxiety, irritability, delirium, and depression.1In addition to amphetamine, methylphenidate is used to treat the EDS associated with narcolepsy. Methylphenidate is mostly commonly known as the primary treatment for children with attention-deficit hyperactivity disorder (ADHD).5 The mechanism of action of methylphenidate is much simpler than that of the amphetamines. Methylphenidate binds to the dopamine transporter and prevents it from taking up dopamine. It does not influence the VMAT. Therefore, methylphenidate is solely a dopamine re-uptake inhibitor.16
Methylphenidate is prescribed in doses from 10 to 100mg/day.12 This leads to an increased alertness, but can also create noticeable side effects.3,5 Possible sympathomimetic effects include increased blood pressure and tachycardia. Insomnia is also a likely side effect.
The newest treatment for narcolepsy is sodium oxybate, which both reduces the frequency of cataplexy attacks and decreases associated excessive daytime sleepiness.17
Sodium oxybate is a formulation of g-hydroxybutyrate, an endogenous neurotransmitter and metabolite of gamma aminobutyric acid (GABA), which has complex effects on several neurotransmitter systems.18
While its precise mechanism in cataplexy is unknown, the effects may be mediated in part through interaction with GABA B and gammahydroxybutyric acid (GHB) receptors.19 The recommended starting dose is 4.5g/night, but, due to its very short half-life, it must be taken in two equal doses 1.2 to four hours apart.17 This can be increased for optimum therapeutic response to a maximum dose of 9g/night.
Sodium oxybate is an oral solution containing 0.5 g/ml sodium oxybate and is taken as a liquid at night. A problematic feature of the liquid is the high sodium load, which limits its use in patients with compromised renal and/or cardiac conditions.20
While sodium oxybate is a highly controlled substance (Scheduled III in the US) due to the unfortunate recreational abuse of GHB (a related substance), it has been shown to be safe when taken as directed.17 The most common side effects with sodium oxybate are dizziness, headaches, and nausea. Middle insomnia is also reported. Many unapproved medications have been used for the treatment of cataplexy, including the tricyclic antidepressants such as imipramine, clomipramine, and protriptyline, the newer generation anti-depressants such as venlafaxine, and the ADHD treatment atomoxetine. However, these medications may have significant side effects, including sexual dysfunction and discontinuation syndrome.17
Because narcolepsy can be such a crippling problem, combinations of medications are often warranted. While not formally studied, modafinil may be added to traditional stimulants. As the mechanism of action of these compounds is quite different, this combination is considered ‘rational polypharmacy.’11 In addition, modafinil and sodium oxybate can be combined,21 as can stimulants and sodium oxybate. In fact, in sodium oxybate clinical trials, approximately 80% of patients maintained concomitant stimulant use.22
Because the current treatments for narcolepsy are often inadequate and sometimes cumbersome, many additional agents are under investigation. These include histamine 3 (H3) receptor antagonists such as thioperamide. H3 receptors control the synthesis and release of histamine via a G protein. Stimulation of this central H3 receptor reduces histamine release, so antagonism of this receptor increases histamine release. Increasing histamine results in increased wakefulness in animal models.23 Orexin (hypocretin) and its agonists have generated much excitement in this field. Orexins are ineffective when taken orally. Intense interest has developed for searching for a feasible way to restore orexin to these orexin-deficient individuals. Additionally, ampakines such as CX-717 can increase wakefulness in laboratory models.Non-pharmacological treatment options are available to help narcoleptic patients deal with handling their disorder in today’s society. A primary goal after the diagnosis of narcolepsy is for patients to focus on developing a routine sleep/wake schedule to decrease the effect that EDS has on daily schedules.2 Caffeine, the most popular psychoactive substance in the world, is sometimes recommended for narcoleptic patients to increase alertness.5 However, overconsumption of caffeine can lead to restless nights if taken in high quantities or late in the day, and caffeine withdrawal can cause headaches.5 Contrary to the goal of increased wakefulness, napping multiple times per day for 15–30 minutes at a time may combat instances of ‘sleep attacks.’5 Individual therapy or group sessions are recommended in addition to any treatments to educate an individual on living with the disorder and the chronic nature of narcolepsy, especially for younger individuals.2 These therapy sessions can help to warn the narcoleptic patient of potential risks: sleep attacks while driving, temporary paralysis with the experience of intense emotions, and understanding the reality of often frightening hypnagogic hallucination.1,2
Conclusions
Current understandings of narcolepsy are incomplete. Research reflects a number of potential causes for narcolepsy and currently available treatments do not always produce robust clinical response, and are often accompanied by side effects. Patients suffering from narcolepsy are often misdiagnosed or under-diagnosed due to the co-occurrence of other sleep disorders, each disorder requiring specific treatments.2 Symptoms of narcolepsy affect the quality of life for patients living with the fear of the sudden onset of sleep attacks at inappropriate times. The future of narcolepsy research has many possible routes: investigating lesions in the hypothalamus, observing the abnormalities of brain neurons, further examination of genetic mutations,3,4 immunotherapy, cell transplantation, and plasmapheresis, to name just a few.24 Continued research and potentially improved medications will give the hope of controlling the symptoms of this chronic disease, leading to a higher individual quality of life for narcoleptic patients.