Delirium is a complex neuropsychiatric syndrome characterized by disturbances of consciousness, attention, cognition, and perception. It is a common condition with serious consequences. It is estimated that at admission 10–15% of elderly patients are delirious,1 and 10–40% become delirious during hospitalisation.2 Some groups of patients in particular seem to be at risk for developing a delirium. In postoperative hip fracture patients,3,4 hospitalized patients with dementia,5 patients with Parkinson’s disease,6 and acute stroke patients,7 high incidence figures have been reported. Recognition of delirium is very important as it is potentially reversible and because delirium is associated with increased duration of hospitalization, poor functional status, need for institutional care,8 and higher mortality rates.9
Treatment of delirium consists of three components: identification and correction of the underlying cause(s), environmental and supportive intervention, and symptomatic pharmacological treatment.10,11 With regard to pharmacological treatment, haloperidol is advocated as the drug of first choice and is most commonly used. However, there is some debate regarding the pharmacological treatment of delirium, and several other drugs are used. In this review we discuss the use of an acetylcholinesterase inhibitor, rivastigmine, which is a promising novel drug in the management of delirium.
Pathophysiology of Delirium
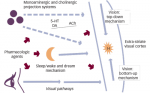
Given the clinical heterogeneity and the multifactorial nature of delirium, it is likely that multiple mechanisms play a role in the pathophysiology of delirium. Since delirium is often associated with infection and other conditions accompanied by stress, glucocorticoids and cytokines are considered to play key roles. Additionally, alterations in the release of neurotransmitter(s) appear to be important.
Stroke, infection, and pain can be considered as stress conditions and may lead to increased glucocorticoid production. In delirium, due to an abnormal shut-off of the hypothalamic–pituitary–adrenal axis,12 this increased glucocorticoid production is not adequately suppressed. This mechanism leads to increased plasma cortisol levels. Corticoids are known to affect mood and disturb attention and declarative memory.13,14 Therefore, hypercortisolism may be a mediator of delirium. In the early phase of stroke, hypercortisolism with decreased suppressibility of dexamethasone has been demonstrated.15–17
In infection there is increased production of cytokines, especially interleukins (IL) (types 1, 2, 6 and 8), tumor necrosis factor-alfa (TNF-α), and interferon (IFN). These cytokines may contribute to delirium by increasing the permeability of the blood–brain barrier and by altering neurotransmission.18–20 In humans, high serum levels of IL-6 and other cytokines have been associated with neuropsychiatric illnesses such as cognitive decline in dementia,21 depression,22 and cognitive impairment and fatigue in cancer23,24 and delirium.25 Animal studies have shown that cytokines can cause a reduction in the acetylcholinergic pathways.26
The main neurochemical correlate of delirium is probably decreased cholinergic activity. The muscarinic cholinergic system has been the focus of much delirium research.27 Acetylcholine plays a role in the level of cortical arousal, attention processes, learning and memory, induction of rapid eye movement (REM) sleep, motor components of behavior, mood, thought, perception, and orientation.28–35 Dysfunction in these processes constitutes the core features of delirium. Anticholinergic drugs, drugs with subtle anticholinergic effects, or drugs that bind to muscarinic receptors can precipitate delirium.36 Patients taking five or more drugs with modest anticholinergic activity had a higher risk of delirium.37 The use of anticholinergic medication was associated with an increase in delirium in elderly medical patients.38 The age-related loss of cholinergic reserve and focal loss of acetylcholine in the nucleus basalis of Meynert together with decreased receptor function may be the reason why delirium is more common in elderly and in patients with dementia.17,39 The increased level of anticholinergic activity found in the serum of patients with delirium also supports the cholinergic deficiency hypothesis.40 This is further corroborated by the observation that physostigmine can reverse delirium associated with anticholinergic drugs, and that cholinesterase inhibitors appear to have some benefit even in cases of delirium that are not induced by drugs.19,38,41
Dopamine is another neurotransmitter that appears to contribute to delirium. This may be due to its regulatory influence on the release of acetylcholine.19 Dopaminergic drugs, such as levodopa, are recognized precipitants of delirium, and dopaminergic antagonists (for example antipsychotic agents) effectively treat symptoms of delirium.42 Perturbations of other neurotransmitters, such as norepinephrine, serotonin, gamma-aminobutyric acid (GABA), glutamate, and melatonin, may have a role in the pathophysiology of delirium,18–20 possibly through interactions with the cholinergic and dopaminergic pathways.42
Pharmacological Properties of Rivastigmine
Rivastigmine is a non-selective inhibitor of central cholinesterases and inhibits both acetylcholinesterase and butyrylcholinesterase, unlike other, acetylcholine-specific esterase inhibitors.43,44 Reduced activity of these enzymes leads to a higher neurotransmitter concentration after pre-synaptic release, thereby amplifying its post-synaptic action.
Butyrylcholinesterase activity is relatively high in thalamic nuclei that project to frontal cortical structures involved in attention, executive function, emotional memory, and behavior.45 Thanks to this feature, its rapid absorption (maximum concentration after 0.5–2 hours), and rapid clearance (0.6–2 hours),43 rivastigmine is theoretically interesting for treatment of delirium.
Rivastigmine for the Prevention of Delirium
Prevention of delirium with rivastigmine was first mentioned in a case report in 2003.46 It describes a 65-year-old patient with Parkinson’s disease since 1988 with no signs of cognitive decline. Two delirious episodes are mentioned: one that contributed to an exacerbation of ulcerative colitis in 1998 and the other to endocarditis in 2000. The first episode was not successfully treated with olanzapine and risperidon over 10 weeks. After starting with rivastigmine (6mg/day), the delirium resolved within two weeks. The second period was treated successfully with rivastigmine at once. Subsequently, this patient needed an aortic valve correction. In order to prevent delirium he started with rivastigmine six weeks prior to the operation (3mg/day augmented to 9mg/day two weeks prior to the operation). During the hospitalization there were no symptoms of delirium, and rivastigmine was tapered six weeks after discharge from hospital.
In a retrospective study,47 11 patients with chronic rivastigmine use were compared with 30 patients randomly selected from a group of 332 geriatric patients. Patients in the chronic rivastigmine group suffered from delirium less frequently than patients in the non-rivastigmine group (45.5% [confidence interval 16.1–74.9] and 74.9% [confidence interval 77.1–100.7], respectively).
There is one prospective study of the prevention of delirium with rivastigmine in a group of outpatients with vascular dementia.48 In this study, 230 patients between 68 and 85 years of age who were ambulatory outpatients were divided into two homogenous groups matched for age, level of education, co-medication, and concomitant disease. Group A (n=115) received rivastigmine 3–6mg/day, while group B (n=115) received aspirin 100mg/day. Delirium was assessed by the Confusion Assessment Method (CAM).49 Patients were visited at one, three, nine, 12, 15, 18, 21, and 24 months. Their mean age was 76.23±4.72 years. In total 48% of the patients (n=117) suffered from a delirium (during medical illnesses such as complications after a fall, sudden hospitalization, or after anesthesia). In group A 40% and in group B 62% (p<0.001) became delirious, with a mean duration of 4.0±1.71 days in group A and 7.86±2.73 days (p<0.01) in group B. In group A, rescue medication (benzodiazepine or neuroleptics) was less often needed for the treatment of delirium (p<0.05).
Rivastigmine for the Treatment of Delirium
In a case report a patient with severe delirium (delirium symptom score50 29/32) after lithium intoxication was described.51 Initially the delirium persisted after normalization of the lithium level, but within 48 hours after starting rivastigmine 1.5mg twice daily (BID), the patient recovered completely (delirium symptom score 8/32).
In a retrospective study52 the effects of rivastigmine in delirious patients not responding to antipsychotics was described. In this study in 21 patients, rivastigmine was added to antipsychotic medication in dosages ranging from 3 to 9mg/day. Recovery of delirium after rivastigmine treatment occurred in 15 of the 21 patients (71.4%). Rivastigmine was also used in three elderly patients with a chronic delirium lasting for four weeks.53 Initially, they were treated with haloperidol for 14 days followed by risperdone for 14 days, without improvement. After four weeks they were treated with rivastigmine. Within one week the symptoms of the delirium cleared with dosages between 6 and 12mg/day. Two of the patients fulfilled the criteria of dementia and rivastigmine was continued. One patient (81 years of age with a subdural hematoma and a humerus fracture) functioned independently after rivastigmine was tapered.
Recently, the feasibility of rivastigmine in the treatment of delirium after stroke was studied.54 In this study, patients (n=17) with a severe and persistent delirium (defined as a delirium rating scale [DRS]55 >12 for more than 24 hours) were treated with rivastigmine. The treatment was initiated at a dose of 1.5mg BID and was raised every other day by 3mg, depending on the clinical response, with a maximum dose of 12mg/day. If the DRS remained below 10, rivastigmine was continued as a fixed dose for one week and then tapered. In 16 patients (94%), the delirium improved. The mean DRS decreased from 14.8 to 8.5 at individual maximum dose and to 5.6 after tapering. Four patients needed 3mg/day rivastigmine, five patients 6mg/day, five patients 9mg/day, and two patients 12mg/day. In all patients it was possible to taper the dose of rivastigmine without recurrence of delirium in the first month. There were no significant side effects.
Unfortunately, no randomized controlled trials concerning the treatment of delirium with rivastigmine have been performed.
Conclusion
Delirium is a common complication in elderly hospitalized patients, which is important to recognize because prompt treatment is indicated. Rivastigmine is a promising drug for the treatment of delirium because of its pharmacological properties and the central role of decreased cholinergic activity in the pathophysiology of delirium. Unfortunately, no definite scientific evidence for the effectiveness of rivastigmine in the treatment of delirium is available.56 Randomized controlled trials to evaluate the efficacy of rivastigmine in the treatment and prevention of delirium are urgently needed. ■