Parkinson’s disease (PD) is a chronic progressive neurodegenerative disorder characterized by the degeneration of nigrostriatal dopaminergic neurons, with its incidence increasing globally.1 With disease progression, the benefit from medications shortens, and symptom control becomes strictly dependent on peripheral levodopa (L-dopa) levels, leading to the development of daily fluctuations of motor and non-motor symptoms severity and L-dopa-induced dyskinesia.2
Advanced PD (APD) is characterized by the emergence of motor complications (e.g. OFF time >2 hours/day and troublesome dyskinesia) and the impairment of functional independence of the affected person despite optimal oral L-dopa therapy.3 Although clinical guidelines have been proposed to define APD, the complexity of this disease phase is being increasingly recognized, especially with respect to the contribution of non-motor symptoms and the impairment of quality of life, implying a broader definition of this disease stage as the “complex phase” of PD.4 At this stage, adjunctive treatment options to L-dopa therapy should be considered, including dopamine agonists, catechol-O-methyltransferase inhibitors and monoamine oxidase-B (MAO-B) inhibitors.5 However, these treatments almost invariably fail to provide long-term control of symptoms, and device-aided therapies (DATs), such as deep brain stimulation (DBS) and infusion therapies, might become the only effective alternative to oral therapy.6
The rationale for L-dopa infusion therapies is to provide constant plasma levels of L-dopa, potentially leading to more stable synaptic concentrations of dopamine and, therefore, continuous stimulation of brain striatal dopamine receptors. In turn, this could lead to the putative maladaptive changes underlying the pathophysiology of symptoms’ fluctuations induced by the pulsatile dopaminergic stimulation associated with oral therapy.7 This therapeutic approach also improves medication tolerability and treatment adherence compared with oral polytherapy.8
Drug delivery systems bypass problems related to the irregular and often unpredictable intestinal absorption of oral L-dopa, which significantly affects its bioavailability.9 In line with this evidence, recent European Academy of Neurology/Movement Disorder Society-European Section guidelines on invasive therapies suggest infusions as valid treatment options in patients with symptoms poorly controlled by oral medications.10 However, the need for specialized healthcare professionals for their management and the cost-effectiveness with respect to oral medications must be taken into account and weighed against the improvement of quality of life and motor and non-motor symptoms of patients with APD. Furthermore, current guidelines refer only to patients receiving levodopa/carbidopa intestinal gel (LCIG), DBS and subcutaneous apomorphine, and no recommendations have been produced yet for subcutaneous L-dopa and L-dopa/entacapone/carbidopa intestinal gel (LECIG).
Currently, there are only two L-dopa formulations available for continuous intestinal infusion. The most broadly used consists of a gel suspension of L-dopa/carbidopa monohydrate (LCIG) delivered into the patient’s proximal small intestine tube placed via percutaneous endoscopic gastro-jejunostomy (PEG-J) connected to a portable pump. Several thousands of patients have been treated with LCIG worldwide; however, the need to perform a percutaneous gastrostomy has significantly limited its broader adoption.11 Patients’ acceptance of the therapy is limited by the psychological and physical discomfort associated with PEG-J and the portable pump, the need for daily system maintenance, and the risk for adverse events. The overall frequency of adverse events is up to 80%; however, it is mainly related to the endoscopic procedure or device-related complications (e.g. stoma infections, tube displacement) while showing a reduction with long-term treatment.12 Furthermore, the PEG-J placement involves a multidisciplinary team, including trained healthcare professionals and dedicated personnel for patient training and follow-up over time, potentially limiting its use in non-specialized centres. Finally, despite compelling evidence of its efficacy on patients’ quality of life and motor and non-motor symptoms, the cost-effective profile of LICG is less favourable with respect to DBS and subcutaneous apomorphine.13 According to the current guidelines, contraindications to LCIG include the presence of dementia (as with other DATs), lack of a caregiver, presence of orthostatic hypotension, dopamine-dysregulation syndrome, gastrointestinal comorbidities, and troublesome non-drug responsive motor and non-motor symptoms.14 Furthermore, no specific age limits are defined for the eligibility for the treatment.
LECIG is a novel infusion treatment consisting of a triple combination of L-dopa, carbidopa and entacapone.15 Similar to LCIG, LECIG is continuously delivered into the duodenum/jejunum through a PEG-J connected to a portable pump. The innovation relates to the addition of the catechol-O-methyltransferase inhibitor entacapone, which blocks L-dopa degradation by reducing its conversion to 3-O-methyldopa, thereby increasing the L-dopa plasma concentration and reducing the total L-dopa administered daily. Moreover, the LECG infusion pump is smaller and lighter with respect to LICG, improving patients’ comfort and device portability. There are currently few studies concerning the efficacy of LECIG.16–18 One such study was a randomized, open-label, crossover, pharmacokinetic study conducted in 11 patients with APD.16 The results showed comparable L-dopa exposure between LCIG and LECIG, with a 20% reduced infusion rate compared with the usual LCIG dose. Importantly, treatment response scale scores were similar between treatments. In this study, adverse events were described in 55% of patients, they were mild and did not lead to study discontinuation; no serious side effects were reported.16 However, given the small size of the population and the short time of the observation, more studies are needed to confirm these results and provide long-term safety data. A large observational multinational phase II study, named ELEGANCE (ClinicalTrials.gov identifier: NCT05043103), is ongoing and will provide real-life evidence of long-term efficacy with over 1-year follow-up in almost 300 patients with APD.15
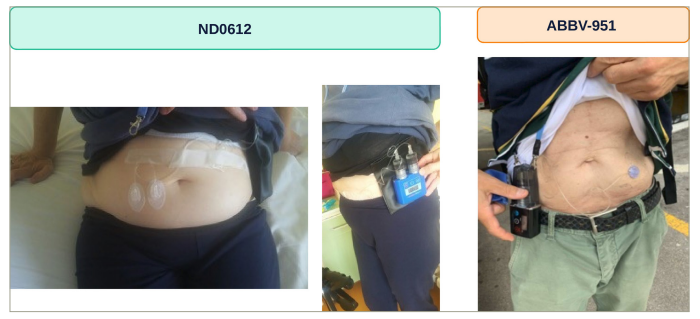
Upcoming subcutaneous levodopa infusion systems
Two soluble formulations of L-dopa/carbidopa have been developed in recent years for continuous subcutaneous infusion (CSCI), which can be delivered by a portable pump infusion system such as that used for apomorphine infusion. The two compounds, ND0612 (NeuroDerm, Rehovot, Israel) and ABBV-951 (AbbVie Inc., North Chicago, IL, USA), are close to being introduced to global markets (Figure 1).
Figure 1: Upcoming infusion delivery systems for continuous subcutaneous levodopa/carbidopa therapy
ND0612 consists of a solution of L-dopa/carbidopa, which was shown to provide stable and therapeutic plasma concentrations of L-dopa in different studies.19,20 When used as an add-on therapy to oral treatment, continuous delivery of ND0612 was shown to reduce the variability and abolish deep troughs in L-dopa plasma levels compared with placebo.20 The first evidence of the efficacy and safety of ND0612 derives from an open-label study (ClinicalTrials.gov identifier: NCT02577523) where patients with APD showed a decrease in OFF time by approximately 2.0 hours and increased ON time without troublesome dyskinesia by approximately 3.3 hours.21 Patients in the 24-hour infusion group demonstrated a greater reduction in OFF time compared with patients receiving the 14-hour infusion. Recently, results from the double-blind, double-dummy phase III trial BouNDless (ClinicalTrials.gov identifier: NCT04006210) were announced, reporting a significant extension of 1.72 hours in ON time without dyskinesia and a small but significant reduction in OFF time (3.4 hours in the ND0612 group compared with 3.7 hours in the oral L-dopa group).22 Furthermore, as secondary endpoints of the study, the therapy achieved improvement in clinical scales scores, evaluating the impact of motor symptoms in daily activities and the patients’ self-perceived clinical improvement. In an open-label long-term safety study (Beyond trial; ClinicalTrials.gov identifier: NCT02726386), adverse effects were reported in 73.9% of patients in the first year and 39.4% after the third year of ND0612 treatment and consisted mainly of infusion-site reactions (60.9%), including infections, haematomas and skin nodules.23 Local skin adverse events caused study discontinuation in 10.3% of patients in the first year but were mild to moderate in most cases.24
ABBV-951 is a new soluble formulation of L-dopa and carbidopa prodrugs, foslevodopa/foscarbidopa, which can be delivered subcutaneously for 24 hours/day. After administration, foslevodopa/foscarbidopa is quickly converted to L-dopa/carbidopa via alkaline phosphatases, reaching and maintaining therapeutic steady-state plasma levels in a very short time. Clinical studies have demonstrated that stable L-dopa plasma levels can be achieved for 24 hours.25,26 Moreover, in patients switched from LCIG, the magnitude of L-dopa plasma fluctuations in the first 16 hours of infusion was comparably low for both treatments, suggesting that foslevodopa/foscarbidopa CSCI maintains L-dopa exposure within a narrow therapeutic window like LCIG.27 A pivotal study on the efficacy and safety of foslevodopa/foscarbidopa CSCI has recently been published.26 In this multicentre, double-blind, double-dummy, active-controlled phase III trial (ClinicalTrials.gov identifier: NCT04380142), 141 patients with APD inadequately controlled by the optimal medical therapy were randomized to receive either foslevodopa/foscarbidopa CSCI and oral placebo or CSCI placebo and oral L-dopa/carbidopa.26 After 12 weeks of treatment, patients receiving foslevodopa/foscarbidopa showed a significantly greater decrease in OFF time (mean reduction of -1.79 hours) and an increase in the ON time without troublesome dyskinesia (mean reduction of 1.75 hours) compared with the group receiving oral L-dopa/carbidopa. Concerning safety outcomes and adverse events, most were non-serious and mild-to-moderate in severity. The most frequent adverse events were infusion site reactions (72%), and adverse events that led to premature study drug discontinuation (22%).26
Selecting a DAT for individuals in advanced stages of PD is a complex process that necessitates collaboration between various medical disciplines and the involvement of patients and their support network. Therefore, adopting a shared decision-making approach becomes imperative. While the course of the treatment must be tailored to the individual, certain general principles can offer guidance when considering DATs. These principles should take into account factors such as the patient’s age, cognitive status, presence of dyskinesia, frailty and individual preferences.28 Notably, the need for daily care of the delivery systems for L-dopa intestinal or subcutaneous infusions requires the ability of the patient to receive training on the use of the device and the presence of a caregiver or a support network. Therefore, the patient’s compliance and cognitive status should be taken into account when considering infusion therapies. On the other hand, considering the lower surgical risks with respect to DBS, infusion therapies may be of choice in older or frailer patients.10 Furthermore, the short- and long-term cost-effectiveness of L-dopa subcutaneous infusions and LECIG are still lacking and will need to be compared with the other available DATs.
Conclusion
Gaining an understanding of the relative advantages offered by each treatment option provides supplementary insights that can assist patients, caregivers and healthcare providers in making an informed choice regarding the most suitable therapy. This choice aims to ensure optimal management of symptoms and an enhanced quality of life.29 However, current guidelines may not provide a clear-cut indication for a specific treatment at the single-patient level, and patients may be suitable for more than one treatment or may be switched from one DAT to another over time.30 In the future, given the increase in surgical and infusion therapeutic options, it will be crucial to concentrate on identifying potential candidates for DATs at earlier stages, increasing appropriate referrals and expanding the availability of these therapies on a global scale.8 This expansion also holds the promise of widening access to costly treatments for individuals with APD in developing world regions.31