Case study
Patient information
A 42-year-old woman presented in the emergency department with acute onset whole-body myoclonic jerks for 1 day. On enquiry, the patient’s parents advised that she had a history of depression over the past 15 years. Intermittently, family members had also noticed aggressive and abusive behaviour. She had been evaluated and diagnosed with hypothyroidism. She was on irregular medication for depression and hypothyroidism.
Informed consent
Written consent for the publication of this case report was obtained from the parents of the patient.
Clinical findings
The patient was conscious, oriented and alert. At rest, multifocal, mild-to-moderate myoclonus was detected in all four extremities; this was dramatically worsened by muscular activation. Reflex sensitivity was present. The rest of the neurologic evaluation was uneventful. She had a score of 21 on the Hamilton Depression Rating Scale (HAM-D).
Diagnostic assessment
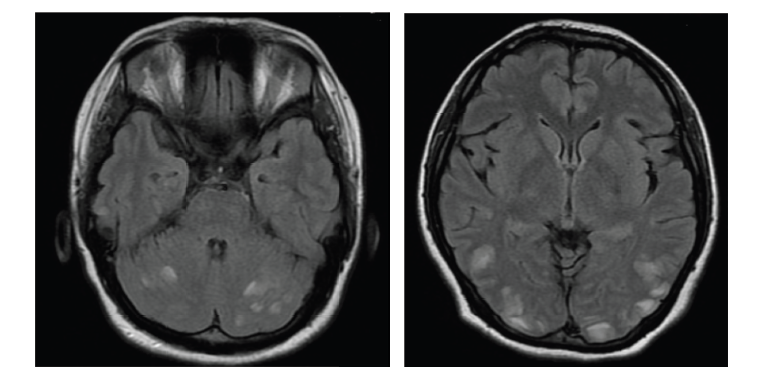
The patient’s laboratory evaluation showed elevated antithyroid autoantibodies. Antithyroglobulin (anti-TG) antibody level was >2000 IU/mL and the antithyroid peroxidase autoantibody (anti-TPOAb) level was >2,000 IU/mL. Thyroid function test showed a thyroid stimulating hormone (TSH) value of 5.65 mIU/mL; a free triiodothyronine value of 3.36 pg/mL and a free thyroxine value of 1.02 ng/dL. The patient had a vitamin B12 level of 6,764 pg/mL, a folate level of 8 ng/mL and a vitamin D3 level of 121 pmol/L. Her cholesterol level was 244 mg/dL, with a low-density lipoprotein level of 156 mg/dL and normal triglyceride level. The antinuclear antibody and extractable nuclear antigen profiles were negative. Cytoplasmic antineutrophil cytoplasmic antibodies and perinuclear antineutrophil cytoplasmic antibodies were negative. The serum test for autoimmune encephalitis panel was also negative. The neck ultrasonography showed a diffusely enlarged thyroid gland with evidence of calcification, haemorrhage and necrosis. Brain magnetic resonance imaging (MRI) was normal. The interictal electroencephalogram (EEG) findings were normal. The patient was diagnosed with SREAT after meeting the diagnostic criteria points 1–6 of the proposed steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT) diagnostic criteria:1
-
Encephalopathy with seizures, myoclonus, hallucinations or stroke-like episodes.
-
Subclinical or mild overt thyroid disease (usually hypothyroidism).
-
Brain MRI normal or with non-specific abnormalities.
-
Presence of serum thyroid (thyroid peroxidase, thyroglobulin) antibodies.
-
Absence of well-characterized neuronal antibodies in serum and cerebrospinal fluid (CSF).
-
Reasonable exclusion of alternative causes.
Therapeutic intervention
The patient was treated with a high dose of methylprednisolone (1 gm/day) for the first 5 days, followed by a dose of oral prednisolone of 60 mg.
Follow-ups and outcomes
The patient’s myoclonic jerks disappeared after she started taking pulse dose of methylprednisolone, and her depression symptoms also improved. A psychiatric evaluation was obtained for depression, and sertraline 100 mg tablet twice daily and risperidone 1 mg twice daily were prescribed. Her HAM-D rating score at the time of discharge was 12.
Discussion
SREAT (also called Hashimoto’s encephalopathy [HE] or Hashimoto’s thyroiditis), which was first described in 1966, is still a contentious and poorly understood condition.2 SREAT is thought to be a neuroimmunological illness rather than a direct effect of thyroid dysfunction on the central nervous system.
The exact pathophysiology of SREAT remains unclear, and several pathophysiologies have been hypothesized.3 SREAT may represent an immune complex disease or a primary demyelinating process, such as acute disseminated encephalomyelitis.4–6 It may result from a direct antibody neuronal injury,7 or vasculitis from endothelial inflammation or immune complex deposition.8
In line with most autoimmune disorders being more common in women, SREAT is also more common in women.9
Acute-to-subacute onset of altered consciousness is the most common clinical symptom of SREAT. Focal or generalized tonic-clonic seizures are a common SREAT manifestation, affecting around one-half to two-thirds of patients.10,11 Status epilepticus has been reported in 25% of these patients. Approximately 38% of patients have focal or multifocal myoclonus. Among patients with SREAT, 25% can manifest multiple, recurrent, stroke-like focal neurological deficits.12 SREAT can also have a limbic encephalitis-like presentation, with subacute onset memory impairments, decreased level of consciousness, seizures and behavioural abnormalities.13
SREAT can also have a chronic course, which is marked by slowly growing cognitive impairments, somnolence and confusion.14 Psychosis, specifically visual hallucinations but also paranoid delusions, has been recorded in 25–36% of patients with SREAT. Among individuals with SREAT, 10–25% may present with isolated psychiatric symptoms such as depression, delusion or hallucination.10
Laboratory features
An essential criterion for SREAT is an elevated serum level of anti-TPOAb and/or anti-TG. A systematic review reported that the median anti-TPOAb level at diagnosis is 900 IU/mL.11 In a review of 105 patients with HE, anti-TPOab were elevated in 100% of patients, and TgAb/anti-TG antibodies were elevated in 48% of them.15 However, antibody titres do not correspond with disease activity. Furthermore, the presence of thyroid antibodies is not specific, as approximately 10% of the general population has raised serum anti-TPOab.16
The role of CSF analysis in SREAT is not entirely established. However, it should be conducted to rule out other common differential diagnoses, as mentioned below. An abnormal CSF analysis with an elevated protein concentration and normal glucose level can be seen in around 80% of SREAT cases. A mild lymphocytic pleocytosis can be seen in 10–25% of patients.5,7 Antithyroid antibodies are infrequently measured in CSF. Additionally, the specificity and sensitivity of antithyroid antibodies in the CSF are unclear.7 Other rare findings that are reported are oligoclonal bands and elevated 14-3-3 protein level.17
Thyroid disease in SREAT ranges from overt hypothyroidism to overt hyperthyroidism. According to a systematic review, most patients remain euthyroid. Subclinical hypothyroidism is the most common abnormality (23–35%), followed by overt hypothyroidism (17–25%). Hyperthyroidism is very rare, and is found in approximately 7% of patients.7,8,18
Non-specific slowing of background activity in the EEG can be seen in 90–98% of patients. Other less common EEG findings that have been reported are focal spikes, sharp waves, triphasic waves and frontal intermittent rhythmic delta activity.18,19 Brain MRI results are usually normal in individuals with SREAT; however, approximately half of patients may demonstrate cerebral atrophy or non-specific T2 signal abnormalities in the subcortical white matter.20 Single-photon emission computed tomography (SPECT) may show focal, multifocal or global hypoperfusion.21 Acute phase reactants such as C-reactive protein and the erythrocyte sedimentation rate can also be raised in some cases. A modest increase of liver enzymes can occur in 12–20% of patients.20
Furthermore, newer techniques, such as event-related potentials and magnetic resonance spectroscopy, can be used to recognize and monitor cognitive functions in patients with SREAT. The N-acetylaspartate/creatine ratio exhibited significant negative correlations with all N200 latencies, whereas the myo-inositol/total creatine ratio exhibited significant positive correlations with P300 latencies, implying that decreased values of the N200 and P300 latencies are associated with metabolic changes in the posterior cingulate cortex.22
Changes in the bioelectrical activity of the brain during the course of SREAT in people with euthyroid sick syndrome may indicate an autoimmune process. In a study of 100 patients with SREAT, 34% had aberrant visual evoked potentials and brainstem auditory evoked potentials.23 There was a link between TSH levels and wave brainstem auditory evoked potentials III-V interpeak latency. In recent years, antibodies against α-enolase have been discovered in the serum and cerebral fluid of SREAT patients as a potential biomarker. The presence of α-enolase in individuals with HE was found in 68% of patients compared with 12% of patients with autoimmune thyroiditis without encephalopathy.24 These antibodies, which are not specific, have been found in patients with Creutzfeldt-Jakob disease and limbic encephalitis.24 Differential diagnoses of SREAT include acute disseminated encephalomyelitis, toxic metabolic encephalopathies, meningoencephalitis, psychiatric disease (depression, anxiety and psychosis), carcinomatous meningitis, paraneoplastic or autoimmune encephalitis, degenerative dementia (Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia), stroke or transient ischemic attack, basilar or hemiplegic migraines, and central nervous system vasculitis.24
Diagnosis
Elevated levels of anti-TPOAb or TgAb/anti-TG antibodies with a compatible clinical presentation and a response to glucocorticoids generally define the diagnosis of SREAT. Thyroid hormone levels are variable, with euthyroidism being the most common.
Proposed diagnostic criteria
Diagnosis can be made when all six of the following criteria have been met:1
-
Encephalopathy with seizures, myoclonus, hallucinations or stroke-like episodes
-
Subclinical or mild overt thyroid disease (usually hypothyroidism)
-
Normal brain MRI or with non-specific abnormalities
-
Presence of serum thyroid (thyroid peroxidase, thyroglobulin) antibodies.
-
Absence of well-characterized neuronal antibodies in serum and CSF.
-
Reasonable exclusion of alternative causes.
Treatment
Glucocorticoids remain the standard of treatment for SREAT. Pulse methylprednisolone is usually administered, followed by oral steroids.9,11 Symptoms typically improve over a few months in most patients. However, recovery may be incomplete. According to a series including 24 patients, only 32% of them had a complete response to steroids.11
There is no clear association between serum antibody titres and response to treatment. In addition, antibody levels may or may not decrease following treatment.25
The duration of treatment and rate of taper are generally titrated according to the clinical response. Treatment with steroid-sparing agents, including azathioprine, mycophenolate mofetil, cyclophosphamide and rituximab, should be considered in patients not responding to steroids.20,26–30 The roles of intravenous immune globulin and plasmapheresis have been described in individual cases.28–30 Treatment of a dysthyroid state also goes in parallel, and antiseizure medications may be necessary as a temporary measure.
Prognosis
Most patients remain in remission after discontinuation of steroids over several years of follow-up. A complete or partial neurologic response has been reported in 93% of cases three months after the initial diagnosis. Relapses are seen among 16% of patients over a median follow-up of 1 year.11,30 However, some patients may need long-term immunosuppression.30
Patient perspective
The patient said: “For a long period, I struggled with depression and hypothyroidism. I was in with a seizure and was in a coma. Thanks to the treatment, I was able to improve my symptoms and stop having seizures.”
Conclusion
SREAT is a treatable disorder that can show with chronic neuropsychiatric symptoms such as seizures and myoclonus. This case study emphasizes the necessity of screening people with depression and thyroid disorders for serum antithyroid antibody levels, which aid in the early detection and treatment of SREAT. In most cases, steroid therapy is beneficial.