The prevalence of unruptured intracranial aneurysms (IAs) is approximately 3% of the population, with incidence on the rise due to the increased utilization of neuro-imaging for diverse objectives.1,2 The average risk of rupture for unruptured IA is estimated to vary from 0.3% to exceeding 15% per 5 years.3 Ruptured IA is the primary aetiology of subarachnoid haemorrhage (SAH), resulting in a mortality rate ranging between 40 and 50% and a morbidity rate of 10–20%.4 Based on various studies conducted in Indonesia, ruptured aneurysmal SAH has been documented with a mortality rate ranging from 20.8 to 53.1%. Notably, this mortality rate is significantly elevated when compared with other countries in Southeast Asia.5
Variables indicating a heightened likelihood of aneurysm rupture include female sex, age, smoking and hypertension.6,7 Further factors potentially indicating aneurysm rupture include tobacco use, alcohol intake, diabetes mellitus, heart disease, dyslipidaemia and a familial SAH history, yet clarity on these associations remains uncertain.6,8,9 Various morphological parameters may contribute to an increase in the risk of aneurysm rupture, such as size, irregular shape, number of aneurysms, parent vessel curvatures, vessel type (sidewall or bifurcation), daughter sac and bottle-neck ratio (height/width).9–11
Understanding the predictors of ruptured IA is pivotal for refining risk assessment and devising effective management strategies for patients with IA. This understanding holds promise for substantially enhancing patient outcomes and guiding preventive measures in high-risk individuals. Therefore, this meta-analysis seeks to assess the predictive capacity of these factors, including morphological traits, in clinical conditions.
Methods
Study registration
This meta-analysis has been registered in the International Prospective Register of Systematic Reviews under registration number CRD42023447832.
Search strategy
The methodology of this meta-analysis adhered to criteria established based on the Preferred Reporting Items for Systematic Review and Meta-Analysis 2020 checklist.12 We performed a comprehensive systematic literature search of literature databases, including PubMed, SagePub, Cochrane Library, ScienceDirect and ProQuest, to identify relevant studies published from January 2013 to June 2023. The search terms employed comprised a blend of (‘clinical’ OR ‘morphological’) AND (‘predictor’ OR ‘risk factor’) AND (‘ruptured intracranial aneurysm’ OR ‘intracranial aneurysm rupture’). The data search was not limited by country or language restrictions. Moreover, a manual screening of reference lists of relevant articles was conducted to identify any potential additional articles.
Inclusion and exclusion criteria
Eligible studies were chosen based on the following inclusion criteria: (1) Original studies, including cross-sectional, case–control, cohort studies or randomized controlled trials; (2) patients diagnosed with IA through imaging modalities; (3) inclusion of studies reporting rupture or unruptured aneurysms as an outcome and (4) presenting estimates for aneurysm rupture or enabling their calculation. The following exclusion criteria were implemented: (1) review articles, case reports, editorials, letters and conference abstracts; (2) research involving non-human participants; (3) studies lacking sufficient data for estimating odds ratios (ORs) or weighted mean difference (WMD), alongside corresponding 95% confidence intervals (CIs) and (4) studies with redundant content or duplicates.
Data extraction
Two authors independently conducted data extraction from the relevant studies. Any disparities in decisions were resolved through discussion with the supervisor, who was the third author of the study. The included studies underwent data extraction, retrieving the following information: first author’s name, publication year, country of origin, study design, sample size, gender distribution, age distribution, presence of hypertension, history of smoking, history of alcohol use, presence of diabetes mellitus, presence of dyslipidaemia, history of heart disease, family history of SAH, aneurysm size, aneurysm shape, aneurysm count, parent artery configuration, presence of daughter sac, bottle-neck ratio and outcome event.
Quality assessment
Two authors independently conducted the quality assessment of the included studies. Any discrepancies in their assessments were resolved through discussion with the third author. Each included study underwent systematic quality assessment using the Newcastle-Ottawa Scale (NOS).13 The NOS score, ranging from 0 to 9, considers three aspects: selection (0–4 points), comparability (0–2 points) and outcome (0–3 points). Studies achieving a total score of 7 points were classified as high quality, while those scoring 5–6 points were deemed moderate quality. Studies with a total score of ≤4 points were classified as low quality.
Statistical analysis
Review Manager 5.4 software (The Nordic Cochrane Centre, Copenhagen) was employed for this meta-analysis.14 The association between clinical and morphological risk factors and rupture risk was assessed by calculating ORs and their corresponding 95% CIs using the Mantel–Haenszel method.15 The heterogeneity within the included studies was assessed using Cochran’s Q chi-square test and the I2 statistic.16 A fixed-effects model was used to calculate the pooled ORs and 95% CIs in cases where p≥0.05 and I2≤50%, signifying no significant heterogeneity. If the p-value was less than 0.05 or the I2 statistic exceeded 50%, a random-effects model was used to compute the pooled ORs due to significant heterogeneity. A significance level of p-value <0.05 was considered statistically significant for all test statistics. Potential publication bias was evaluated by visually inspecting the funnel plots.
Results
Literature search
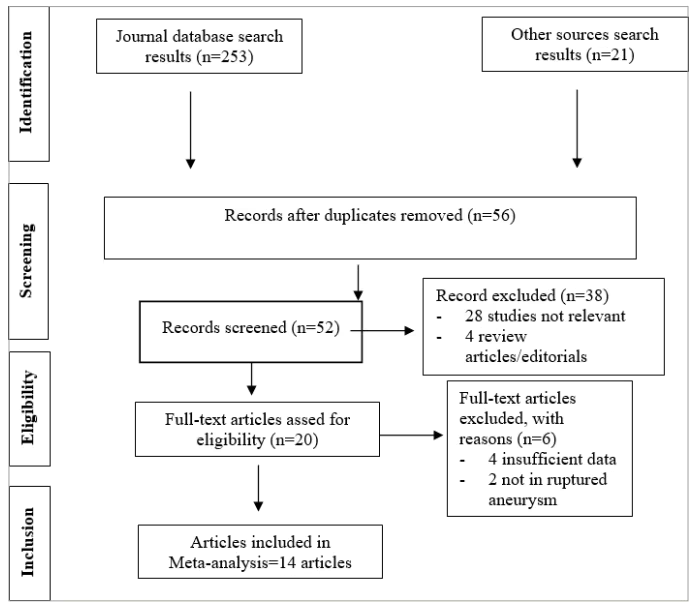
The systematic literature search of electronic databases identified a total of 253 potential articles, supplemented by 21 additional articles identified through manual searching of relevant literature. Following duplicate removal, 56 articles underwent screening based on titles and abstracts. Subsequently, the remaining 52 articles were comprehensively assessed in full text, resulting in the exclusion of 6 articles. Ultimately, our analysis included 14 studies. Figure 1 shows the flowchart delineating the literature search process.
Figure 1: Literature search flowchart
Study characteristic and quality assessment
This meta-analysis included 14 studies, involving a total of 11,546 patients.1,4–6,9–11,17–22 The included studies ranged from 2013 to 2023. Amongst these, three were cross-sectional studies, two were prospective cohort studies, one was a retrospective cohort study, four were case–control studies and four were randomized controlled trial studies. Two studies were carried out in the USA, five in China, one in France, two in the Netherlands, one in the UK, one in Indonesia, one in Spain and one in Korea. The summary of the included studies is shown in Table 1.1,4–6,9–11,17–23
Table 1: Characteristics of the included studies1,4–6,9–11,17–23
| Studies | Origin | Method | Sample size | Mean age (years) | Results |
| Can et al. 201717 | USA | Cross-sectional study | 4,701 patients with intracranial aneurysm | 55.6 ± 13.7 | Ruptured intracranial aneurysms are more frequent in females and are associated with factors such as age, smoking history and alcohol consumption history |
| Lv et al. 201518 | China | Case–control | 89 patients with intracranial aneurysm | 58.7 ± 12 | In terms of clinical characteristics, ruptured intracranial aneurysms were more frequent in females and older patients, and morphologically, they were more frequently associated with larger aneurysms, single aneurysms and a larger bottle-neck ratio |
| Pierot et al. 20196 | France | Cross-sectional study | 1,366 patients with intracranial aneurysm | 54.15 ± 12.55 | Ruptured intracranial aneurysms were associated with hypertension, smoking history and single aneurysm |
| Mocco et al. 201719 | USA | Case–control | 255 patients with intracranial aneurysm | Not reported | Family history of SAH and smoking history more frequently caused intracranial aneurysm rupture |
| Swatan et al. 20225 | Indonesia | Randomized controlled trial | 100 patients with intracranial aneurysm | 51.94 ± 10.78 | Intracranial aneurysm rupture risk is more frequent in females, in older patients and with larger size |
| Tang et al. 202211 | China | Randomized controlled trial | 104 patients with intracranial aneurysm | 63.19 ± 11.0 | In terms of morphological characteristics, intracranial aneurysm rupture risk is higher in bifurcation, irregular shape and a larger bottle-neck ratio |
| Backes et al. 20141 | Netherlands | Prospective cohort study | 302 patients with intracranial aneurysm | Not reported | The irregular shape of aneurysm was associated with aneurysm rupture |
| Bijlenga et al. 201320 | Spain | Cross-sectional study | 932 patients with intracranial aneurysms | 55.02 ± 13.24 | Aneurysm size <7 mm has a higher risk of rupture |
| Wang et al. 20184 | China | Retrospective cohort study | 379 patients with intracranial aneurysms | 57.6 ± 12.88 | Ruptured intracranial aneurysms are more common in females, younger patients and those with alcoholic history, smoking history and multiple aneurysms; the morphological features associated with a higher risk of rupture in intracranial aneurysms are the bifurcation location, daughter sac and an irregular shape |
| Wang et al. 201722 | China | Case–control | 228 patients with intracranial aneurysms | 57.33 ± 11.33 | The irregular shape of intracranial aneurysms increases the risk of rupture |
| Cui et al. 202323 | China | Randomized controlled trial | 204 patients with intracranial aneurysms | 59.90 ± 11.76 | Ruptured intracranial aneurysms are more frequent in females, younger patients, and those with smoking history, alcohol history, hypertension and bifurcation |
| Jeon et al. 201410 | Korea | Randomized controlled trial | 189 patients with intracranial aneurysms | Not reported | In terms of the characteristics of the patient, younger, female and larger size increased the risk of rupture; larger bottle-neck ratio and the daughter sac also increased the risk of rupture |
| Hostettler et al. 20179 | UK | Case–control | 2,334 patients with intracranial aneurysms | 53.2 ± 12.7 | Ruptured intracranial aneurysms are more frequent in females and younger patients and those with a family history of SAH, smoking history, drinking history, hypertension, dyslipidaemia, diabetes mellitus, cardiac disease, larger aneurysms size and multiple aneurysms |
| Koopman et al. 201921 | Netherlands | Prospective cohort study | 409 patients with intracranial aneurysms | 57 ± 13 | The irregular shape and larger aneurysm size increased the risk of rupture |
SAH = subarachnoid haemorrhage.
The NOS was used to evaluate the quality of the included studies. The NOS quality ratings ranged from eight to nine stars, indicating an overall high level of study quality as depicted in Table 2.1,4–6,9–11,17–23
Table 2: Quality assessment of the included studies by Newcastle-Ottawa Scale1,4–6,9–11,17–23
| Studies | Selection | Comparability | Outcome | Total rating |
| Can et al. 201717 | ★★★★★ | ★★ | ★★★ | 9★ |
| Lv et al. 201518 | ★★★★★ | ★★ | ★★ | 8★ |
| Pierot et al. 20196 | ★★★★★ | ★★ | ★★★ | 9★ |
| Mocco et al. 201719 | ★★★★★ | ★★ | ★★ | 8★ |
| Swatan et al. 20225 | ★★★★★ | ★★ | ★★ | 8★ |
| Tang et al. 202211 | ★★★★★ | ★★ | ★★★ | 9★ |
| Backes et al. 20141 | ★★★★★ | ★★ | ★★★ | 9★ |
| Bijlenga et al. 201320 | ★★★★★ | ★★ | ★★ | 8★ |
| Wang et al. 20184 | ★★★★★ | ★★ | ★★ | 8★ |
| Wang et al. 201722 | ★★★★★ | ★★ | ★★ | 8★ |
| Cui et al. 202323 | ★★★★★ | ★★ | ★★ | 8★ |
| Jeon et al. 201410 | ★★★★★ | ★★ | ★★ | 8★ |
| Hostettler et al. 20179 | ★★★★★ | ★★ | ★★★ | 9★ |
| Koopman et al. 201921 | ★★★★★ | ★★ | ★★ | 8★ |
Association between gender and intracranial aneurysm rupture
Ten studies examined the association between gender and IA rupture. The pooled analysis conducted with a random-effects model suggested that there was no significant increase in the risk of IA rupture when comparing females to males (OR=1.01; 95% CI: 0.78–1.30; p<0.93; I2=75%). The forest plot is shown in Figure 2.
Figure 2: Forest plots of the association between clinical risk factors and intracranial aneurysm rupture
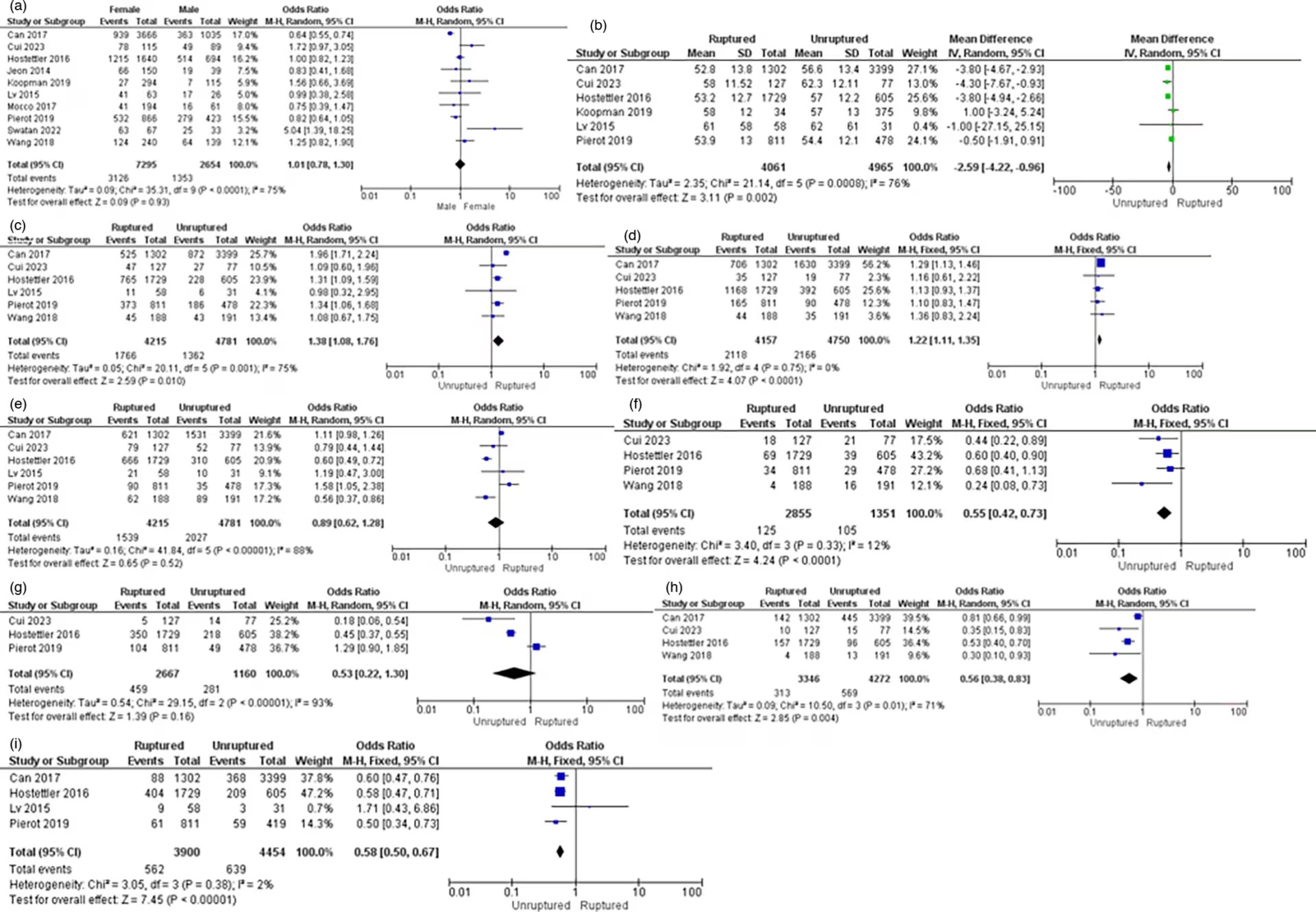
(A) Gender, (B) Age, (C) Smoking history, (D) Alcohol history, (E) Hypertension, (F) Diabetes mellitus, (G) Dyslipidaemia, (H) Heart disease and (I) Family history of subarachnoid haemorrhage.
CI = confidence interval; IV = inverse variance; MH = the Mantel–Haenszel method; SD = standard deviation.
Association between age and intracranial aneurysm rupture
Six studies examined the association between age and IA rupture. The pooled analysis conducted with a random-effects model suggested a higher incidence of IA rupture in younger age categories (WMD=-2.59; 95% CI: -4.22 to -0.96; p=0.002, I2=76%). The forest plot is shown in Figure 2.
Association between smoking history and intracranial aneurysm rupture
Six studies examined the correlation between smoking history and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with a history of smoking had a significantly increased risk of IA rupture (OR=1.38, 95% CI: 1.08−1.76, p=0.010, I2=75%). The forest plot is shown in Figure 2.
Association between alcohol history and intracranial aneurysm rupture
Five studies examined the association between a history of alcohol consumption and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with a history of alcohol consumption had a significantly increased risk of IA rupture (OR=1.22, 95% CI: 1.11−1.35, p<0.0001, I2=0%). The forest plot is shown in Figure 2.
Association between hypertension and intracranial aneurysm rupture
Six studies examined the relationship between hypertension and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with hypertension did not show a significant decrease in the risk of IA rupture (OR=0.89, 95% CI: 0.62−1.28, p=0.52, I2=88%). The forest plot is shown in Figure 2.
Association between diabetes mellitus and intracranial aneurysm rupture
Four studies examined the association between diabetes mellitus and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with diabetes mellitus had a significantly reduced risk of IA rupture (OR=0.55, 95% CI: 0.42−0.73, p<0.0001, I2=12%). The forest plot is shown in Figure 2.
Association between dyslipidaemia and intracranial aneurysm rupture
Four studies examined the association between dyslipidaemia and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with dyslipidaemia did not exhibit a significant decrease in the risk of IA rupture (OR=0.53, 95% CI: 0.22−1.30, p=0.16, I2=93%). The forest plot is shown in Figure 2.
Association between heart disease and intracranial aneurysm rupture
Four studies examined the association between heart disease and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with heart disease had a significantly decreased risk of IA rupture (OR=0.56, 95% CI: 0.38−0.83, p=0.004, I2=71%). The forest plot is shown in Figure 2.
Association between family history of SAH and intracranial aneurysm rupture
Four studies examined the association between a family history of SAH and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with a family history of SAH had a significantly decreased risk of IA rupture (OR=0.58, 95% CI: 0.50−0.67, p<0.00001, I2=2%). The forest plot is shown in Figure 2.
Association between aneurysm shape and intracranial aneurysm rupture
Seven studies examined the association between aneurysm shape and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with irregular aneurysm shape had a significantly increased risk of IA rupture (OR=5.43, 95% CI: 4.62–6.38, p<0.00001, I2=38%). The forest plot is shown in Figure 3.
Figure 3: Forest Plots Displaying The Association Between Morphological Risk Factors and Intracranial Aneurysm Rupture
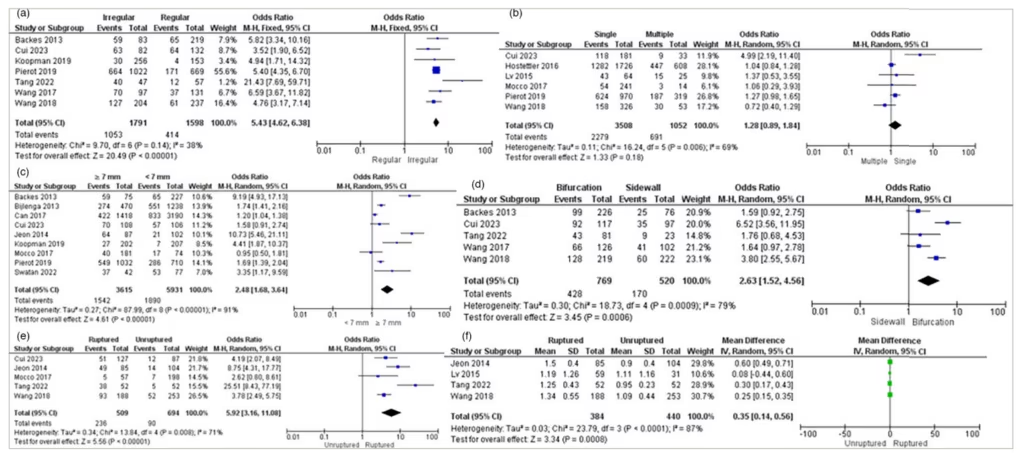
(A) Aneurysm shape, (B) Aneurysm count, (C) Aneurysm size, (D) Parent artery configuration, (E) Daughter sac and (F) Bottle-neck ratio.
CI = confidence interval; IV = inverse variance; MH = the Mantel–Haenszel method; SD = standard deviation.
Association between aneurysm count and intracranial aneurysm rupture
Six studies examined the association between aneurysm count and IA rupture. The pooled analysis conducted with a random-effects model suggested that aneurysm count did not significantly increase the risk of IA rupture. Single aneurysms more commonly increased the risk of rupture (OR=1.28, 95% CI: 0.89–1.84, p=0.18, I2=69%). The forest plot is shown in Figure 3.
Association between aneurysm size and intracranial aneurysm rupture
Nine studies examined the association between aneurysm size and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with an aneurysm size larger than ≥7 mm had a significantly increased risk of IA rupture (OR=2.48, 95% CI: 1.68–3.64, p<0.00001, I2=91%). The forest plot is shown in Figure 3.
Association between parent artery configuration and intracranial aneurysm rupture
Five studies examined the association between parent artery configuration and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with a bifurcation configuration had a significantly increased risk of IA rupture (OR=2.63, 95% CI: 1.52–4.56, p=0.0006, I2=79%). The forest plot is shown in Figure 3.
Association between daughter sac and intracranial aneurysm rupture
Five studies examined the association between a daughter sac and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with an aneurysm with a daughter sac had a significantly increased risk of IA rupture (OR=5.92, 95% CI: 3.16–11.08, p<0.00001, I2=71%). The forest plot is shown in Figure 3.
Association between bottle-neck ratio and intracranial aneurysm rupture
Five studies examined the association between the bottle-neck ratio and IA rupture. The pooled analysis conducted with a random-effects model suggested that patients with a larger bottle-neck ratio had a significantly increased risk of IA rupture (WMD=0.35, 95% CI: 0.14–0.56, p=0.0008, I2=87%). The forest plot is shown in Figure 3.
Publication bias
A funnel plot could not be generated in the meta-analysis due to the limited number of studies available for all risk factor variables, as typically more than 10 studies are required.
Discussion
This systematic review and meta-analysis consisted of 14 studies, including 3 cross-sectional studies, 2 prospective cohort studies, 1 retrospective cohort study, 4 case–control studies and 4 randomized controlled trial studies. The combined study population involved 11,546 patients with IA. Through pooled analysis, we identified clinical and morphological risk factors associated with ruptured IA.
Significant clinical and morphological risk factors associated with an increased risk of ruptured IA include younger age, smoking history, alcohol consumption history, irregular aneurysm shape, larger aneurysm size, aneurysms with bifurcation, aneurysm with a daughter sac and a larger bottle-neck ratio. Conversely, factors associated with a decreased risk of rupture IA include a history of diabetes mellitus, heart disease and a family history of SAH.
Age is a significant risk factor associated with an increasing risk of IA rupture. Cui et al. reported that patients under the age of 50 have a 5.23 times higher risk of IA rupture compared with those aged 50 and above.23 Moreover, in younger patients, specific morphological characteristics, such as the presence of a daughter sac, irregular domes and a larger flow angle, amongst others, further increase the risk of ruptures.24
Smoking compromises the integrity of blood vessel walls, making them more vulnerable to rupture.25 Nicotine, present in cigarettes, elevates plasminogen activator inhibitor-1 in human brain-derived endothelial cells and elevates tissue factor levels, a key factor in thrombogenesis that may predispose to aneurysm rupture.26
Alcohol intake is significantly associated with an increased risk of IA rupture.17 Can et al. report that consuming alcohol exceeding 30 g/day is associated with an increased risk of IA rupture.17 This mechanism suggests that ethanol triggers endothelial damage, fostering vascular inflammation and degradation-induced tissue and cell injury. As a result, this leads to elevated hemodynamic stress, potentially resulting in IA rupture.
An irregularly shaped aneurysm presents a greater risk of rupture compared with those with a regular shape.1 Wang et al. reported that an irregularly shaped aneurysm could disrupt the blood flow pattern, potentially increasing the risk of rupture.4 An aneurysm measuring ≥7 mm in size is associated with an increased risk of rupture. An aneurysm height of ≥7 mm was associated with a higher risk of rupture compared with a height of <7 mm.27 IA located at bifurcations are more frequently associated with rupture than those located at the side wall.4,23 The bifurcation area of arteries is recognized as a susceptible site, distinguished by a weak wall and modified haemodynamic stress.4
A daughter sac refers to an irregular bulge of the IA wall.27 Observational data from Japan indicated that aneurysms with a daughter sac have higher rates of rupture.1 Daughter sacs represent slender segments of the vessel wall susceptible to rupture.28 The bottle-neck ratio was determined by evaluating the ratio between the maximal width and the size of the aneurysmal neck.10 A larger bottle-neck ratio is associated with an elevated risk of rupture.29
The clinical and morphological risk factors that did not exhibit a significant association with aneurysm rupture include female gender and aneurysm count. Multiple studies have reported that females may be at a higher risk of ruptured IA, potentially associated with oestrogen receptors after menopause. This hormonal change could contribute to vascular degradation and reduced fibrillar collagen in the cerebral arteries.30 Patients with unruptured IA have a higher prevalence of multiple aneurysms compared with those with ruptured aneurysms.6
Interestingly, several variables showed a decreased risk of aneurysm rupture, such as a history of diabetes mellitus, heart disease, family history of SAH, hypertension and dyslipidaemia. Hypertension is considered a risk factor for IA. Current hypertension treatments aim to reduce the long-term rupture risk, even in case of a sudden increase in blood pressure.25
Cui et al. reported an inverse relationship between diabetes mellitus and ruptured IA.23 Patients with well-managed diabetes mellitus and a healthy lifestyle reduced the risk of IA rupture. Diabetes induces atherosclerotic alterations, leading to the stiffening of vessel walls, making them less susceptible to dilation and subsequent rupture.31
Cheng et al. reported that dyslipidaemia could significantly reduce the risk of IA rupture.32 However, the use of statins did not seem to affect the risk of IA rupture.32 Dyslipidaemia might offer a protective effect against aneurysm rupture.9 This protective mechanism operates by stabilizing the aneurysm wall through the development of rigid atherosclerotic plaque, consequently averting the rupture of newly formed aneurysms.33 Patients with heart disease showed a lowered risk of intracranial rupture. This phenomenon may be associated with the consumption of aspirin by patients with heart disease. Aspirin functions by inhibiting inflammatory mediators such as matrix metalloproteinases and tumour necrosis factor-alpha.9 A family history of SAH is more frequently found in patients with unruptured IA than in those with ruptured IA.6
Study limitations
Our meta-analysis encountered several limitations. First, the majority of studies included in our analysis were conducted in China, potentially restricting the applicability of our findings to the global population. Second, the variability in sample sizes amongst studies could influence the heterogeneity of the study variables. Finally, due to the limited number of included studies, a funnel plot could not be generated in our meta-analysis, thereby preventing the assessment of potential publication bias.
Conclusion
The systematic review and meta-analysis showed that younger age, smoking history and alcohol consumption history are clinical risk factors associated with an elevated risk of rupture in patients with IA. Morphological risk factors contributing to an increased risk of ruptured IA include irregular shape, larger size, bifurcation, daughter sac and a larger bottle-neck ratio. Conversely, diabetes mellitus, heart disease and a family history of SAH were identified as factors associated with a decreased risk of ruptured IA. Therefore, careful monitoring is recommended for these high-risk patients.
Highlights
-
Subarachnoid haemorrhage (SAH) is caused by ruptured intracranial aneurysm (IA), resulting in a mortality rate between 40 and 50% and morbidity rate of 10–20%.
-
The clinical risk factors include younger age, smoking history, alcohol consumption history and increased risk of IA rupture.
-
The morphological risk factors, such as irregular aneurysm shape, larger bottle-neck ratio, larger aneurysm size, aneurysm with bifurcation and aneurysm with daughter sac, increase the risk of IA rupture.
-
The clinical risk factors such as controlled diabetes mellitus, controlled heart disease and family history of SAH, dyslipidaemia and controlled hypertension, decrease the risk of IA rupture.