Diabetic striatopathy (DS) is a rare hyperglycaemic condition associated with one or both of the following criteria: (1) acute-onset chorea–ballism (random, flowing and nonsuppressible involuntary movements) and (2) striatal hyperdensity on computed tomography (CT) scan or T1-weighted magnetic resonance imaging (MRI).1,2 DS is generally considered a complication of poorly controlled non-ketotic hyperglycaemia with acute hyperglycaemic surge, although it can also be the first presentation of previously undiagnosed diabetes.1,2
It has a prevalence of 1 in 100,000.3 We report two atypical cases of DS in which the only symptom was a substantial decrease in awareness, with no involuntary movements observed. Management of blood glucose levels using insulin led to cognitive recovery in both patients. Therefore, these typical imaging characteristics combined with a hyperglycaemic state can lead to this diagnosis, avoiding unnecessary further investigations.
Case 1
An 82-year-old woman presented to the emergency department with drowsiness for 4–5 days. Her medical history was significant for chronic hypertension and hyperlipidaemia, and she also had at least a 10-year history of diabetes mellitus.
Her medications included fenofibrate, trimetazidine, insulin glargine and lispro.
On physical examination, her body temperature was 36.4 °C, blood pressure was 185/68 mmHg and oxygen saturation was 98%. Her other vital signs were within normal limits.
Neurological examination showed that she was moderately conscious, tended to sleep, oriented and partially cooperative. Her extraocular muscles were intact, with normal-amplitude eye movements in all directions, without nystagmus. The pupils were bilaterally reactive to light with normal accommodation. The rest of the cranial nerve examination was normal. No involuntary movements were observed in her extremities. No athetotic or twisting movements were noted.
At the time of admission to the emergency department, the patient’s glucose level was 232 mg/dL (normal range: 70–115 mg/dL), and her haemoglobin A1C was greater than 14% (normal range: 3.8–5.6%). Urine ketones were also normal.
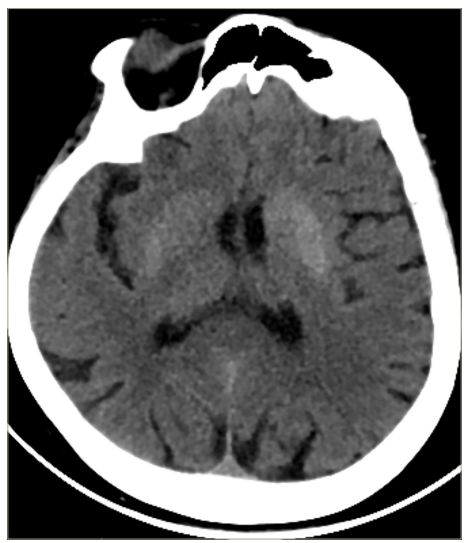
According to the medical anamnesis, she had been admitted to another hospital 2 days prior due to uncontrolled blood sugar. Due to hyperglycaemic laboratory results and poor control of diabetes, hospitalization was recommended, but the patient declined it. A brain CT scan from 2 days prior showed an enhanced hyperdense area in the left putamen and caudate nucleus (Figure 1).
Figure 1: Brain computed tomography scan showing a hyperdense area in the left putamen and caudate nucleus
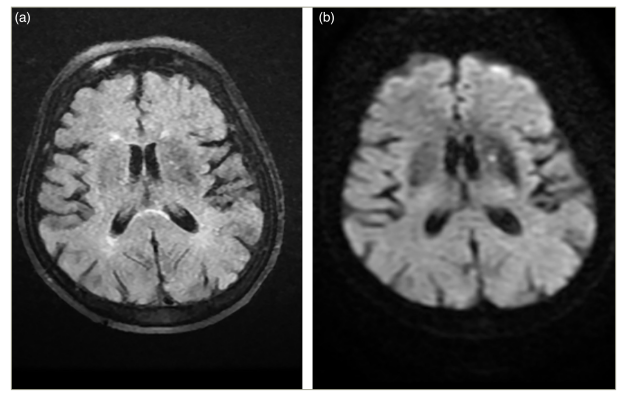
After a 2-day period, the patient’s brain CT scan still showed hyperdense signals in the putamen lesion (Figure 2) and left caudate nucleus. In the fluid-attenuated inversion recovery sequence, mild hypointensity in the basal ganglia and diffusion restriction were observed (Figure 3a, b).
Figure 2: Brain computed tomography showing hyperdensity in the left putamen
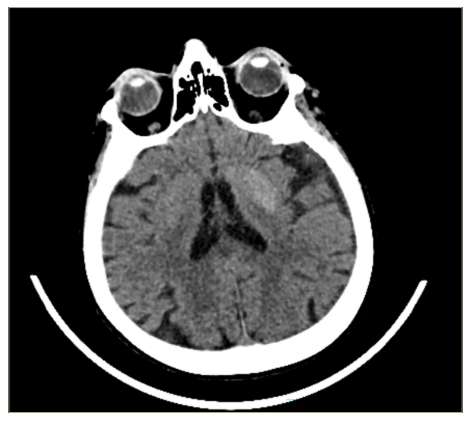
Figure 3: (a) Mild hypointensity in the left basal ganglia in the fluid-attenuated inversion recovery sequence and (b) mild hyperintensity in the left basal ganglia in the diffusion-weighted imaging sequence
According to the patient’s clinical history of poorly controlled diabetes and the unique brain CT findings, DS was diagnosed. The patient was referred to an endocrinologist for blood sugar management. During the hospital stay, her insulin regimen was adjusted, and her consciousness improved by day 2. When she was discharged on day 5, her state of consciousness had recovered.
Case 2
A 47-year-old woman presented to the emergency department with complaints of weakness and an altered state of consciousness that began 2 days ago. During this period, she experienced episodes of blank staring and an inability to respond immediately when questioned.
Her known medical conditions include diabetes mellitus and hypertension.
She had been using insulin glargine 24 units per day, dapagliflozin 10 mg twice daily, vildagliptin 50 mg and metformin hydrochloride 1,000 mg twice daily. She had stopped using oral anti-diabetic medications and insulin without the doctor’s supervision.
On physical examination, she was moderately conscious, tended to sleep and was partially oriented and cooperative. Her cranial nerve examination was normal. There were no obvious signs of paralysis in her extremities. Additionally, there were no extensor plantar responses. No involuntary dyskinesias or abnormal movements were observed in her extremities.
Upon presentation to the emergency department, her glucose level was 550 mg/dL (normal range: 70–115 mg/dL) and her HbA1c was 16.2. Urinalysis showed 2+ ketones, and her sodium level was 129. She was diagnosed with diabetic ketoacidosis.
According to the medical history obtained, the patient had previously presented to another hospital with similar complaints, where a blood sugar level of 612 mg/dL was detected, and she was discharged after blood sugar regulation.
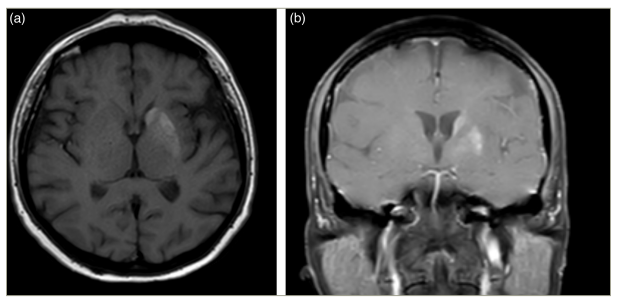
Imaging studies revealed hyperdensity in the left striatum on the CT scan (Figure 4) and hyperintensity in the left caudate nucleus and left putamen on T1-weighted sequences (Figure 5a, b) and signal voids on susceptibility-weighted imaging sequences.
Figure 4: Brain computed tomography showing mild hyperdensity in the left striatum
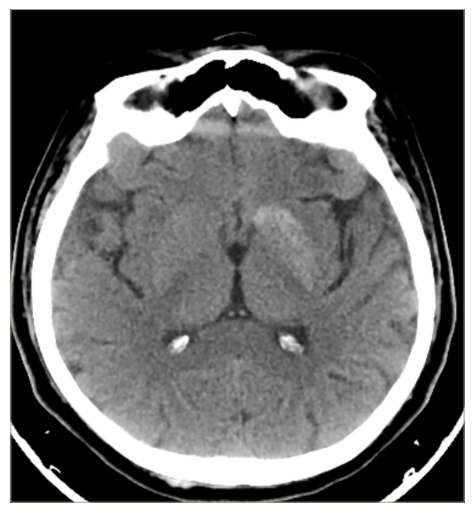
Figure 5: Hyperintensity in the left caudate nucleus and left putamen on T1-weighted sequences in both axial and coronal images
During the hospital stay, the patient was consulted by the endocrinology clinic, and her anti-diabetic treatment was adjusted before discharge.
Therapeutic intervention
The first patient was given an insulin infusion at the hospital where she was first admitted to. The dosage was unknown.
The second patient was diagnosed with diabetic ketoacidosis, and she was started on a 1 U/kg/h insulin infusion with intravenous hydration.
Due to uncontrolled diabetes, the insulin regimens for both patients were adjusted. The dosages were increased. The first patient’s regimen was adjusted to 8 units of insulin aspart three times a day and 24 units of insulin glargine before bedtime. The second patient’s insulin glargine dosage was increased from 12 to 16 units.
Follow-ups and outcomes
With this new insulin regimen, both patients sustained normoglycaemia, and no further neurological deterioration occurred. At the follow-up examination 1 month after discharge, both patients scored 19 out of 20 on the Mini–Mental State Examination.
DS, also known as ‘hyperglycaemic non-ketotic hemichorea or hemiballism’, was first used to describe an uncommon hyperglycaemic condition associated with chorea or ballism and unique reversible abnormality of the basal ganglia on CT scan and/or MRI.4 Few retrospective analyses showed that the prevalence of DS was 1% or even less.5,6
DS is diagnosed after excluding all possible aetiologies capable of causing similar clinical or radiological phenotypes. To refine the list of differential diagnoses, it is important to consider the factors that can cause T1 hyperintensities in the striatum on brain MRI. T1 hyperintensities may result from several factors, including melanin, methaemoglobin in subacute haemorrhage, fat, slow-velocity blood flow, high protein content and paramagnetic transition metals, such as manganese, iron, zinc and copper.7 The clinical presentation of DS can help narrow down these potential causes. For example, copper and iron can be ruled out because copper-deposition disorders, such as Wilson’s disease and neuroferritinopathies associated with pantothenate kinase, typically present as chronic conditions with a gradual onset. Additionally, manganese-deposition diseases can be excluded, as they generally cause parkinsonian symptoms, and haemorrhages tend to present with acute focal neurological deficits.8
DS is usually encountered in the hyperosmolar hyperglycaemic state but less often in diabetic ketoacidosis.9
The pathology is most likely caused by swollen astrocytes known as gemistocytes.10 Among the various pathophysiological theories for DS, Dubey et al. proposed an ‘ominous octet’ model, emphasizing eight factors: (1) gemistocytopathy, (2) petechial haemorrhage, (3) methaemoglobin deposition, (4) mineral (calcium and magnesium) deposition, (5) cytotoxic oedema, (6) myelinolysis, (7) gliosis and (8) atrophy. This model suggests that hyperglycaemia leads to hyperosmolarity and increased blood viscosity, which reduce cerebral blood flow and harm striatal astrocytes, making them particularly vulnerable to ischemia.2
Of the three striatal regions, the most commonly involved region was the putamen (78.6%, 99/126), followed by the caudate nucleus (47.6%, 60/126) and the globus pallidus (27.8%, 35/126). The average time for complete resolution of striatal hyperdensities on the CT scan was 60 days. The underlying cause of the striatal hyperintensity observed on T1-weighted MRI and hyperdensity on CT scans in these patients can be attributed to histopathological evidence of petechial haemorrhages, leading to methaemoglobin accumulation.11 In addition, the presence of gemistocytes due to ischaemic events and neuronal dysfunction might partially account for the striatal hyperintensity seen on T1-weighted MRI; thus, myelin destruction shows a high T1 signal on MRI in DS.10 However, this does not explain the hyperdensity on the CT scan.
A hyperglycaemic state results in hyperosmolarity, leading to reduced cerebral blood flow, which may cause ischaemic insult to the astrocytes of basal ganglia. Petechial haemorrhage, causing accumulation of methaemoglobin and the breakdown of the blood–brain barrier, has been considered the cause of hyperdensity on the CT scan.12,13
Most patients (88.1%) present with hemichorea or hemiballism. Although it is a rare presentation, Sato et al. described a case of DS without hyperkinetic movement disorders, where the patient presented only with severely altered consciousness.14
In a review by Chua et al., the majority of DS manifested with chorea or hemichorea; however, there were 4 patients out of 176 patients who presented with conscious disturbances without chorea or ballism.
The putamen receives neuronal input from the motor, premotor and supplementary motor cortex and is engaged in motor functions, whereas the caudate nucleus receives input from the prefrontal cortex and is involved in cognitive and psychiatric functions.15,16 The cognitive dysfunction in these patients may be associated with the involvement of the caudate nucleus.
The main purpose of DS treatment is to control hyperglycaemia and correct the underlying metabolic imbalance. It is important to recognize these imaging characteristics to suspect this diagnosis.17
We report an atypical case of DS where the only symptom was altered consciousness, with no involuntary movements present. Suspecting this diagnosis, prompt management of blood glucose levels using insulin led to the patient’s cognitive recovery.
Conclusion
DS does not always manifest with involuntary movements but can also present with altered consciousness. With typical CT and MRI findings and concomitant hyperglycaemia, DS should be suspected, and prompt treatment should be initiated. Unfortunately, this condition remains relatively unknown among physicians and endocrinologists, leading to delays in diagnosis and potentially worse outcomes. Most of the patients presenting with DS are already diagnosed with diabetes, but it may also be the first presentation of the disease. Therefore, clinicians need to be aware of this possibility to avoid critical delays and should promptly order glucose measurements when treating such patients, regardless of their previous glycaemic history.